The Effects of Clostridium innocuum on the Development of Autism Spectrum Disorder-Like Sociability and Microglial Morphology in BTBR Mice
Grade 11
Presentation
Hypothesis
GF mice monocolonised with ASD-derived C. innocuum (Ci-8) will exhibit decreased tendencies for social interaction, as well as abnormalities in microglial morphology, supporting the hypothesis that C. innocuum is a pathobiont which promotes the development of ASD.
Research
Gut microbiota develop important local physiological functions crucial for the host, including digestion, barrier protection against pathogens, and production of metabolites and vitamins (Jandhyala et al., 2015). The gut microbiota also affect the morphogenesis, development, and function of other peripheral organs, including the brain (Schroeder & Bäckhed, 2016). Dysbiosis, an imbalance in gut microbiota composition, is linked to the development of gastrointestinal (GI) disorders through the weakening of the epithelium (Wei et al., 2021). Intestinal epithelium in a healthy gut maintains gut homeostasis and functions as a physical barrier between gut microbiota and the immune host cells. In leaky gut syndrome caused by dysbiosis, pathobionts and other microbial-derived metabolites are able to translocate across the weakened GI barrier into systemic circulation and activate inflammatory immune responses, disrupting the central nervous system (CNS) functioning through the excessive production of pro-inflammatory cytokine proteins (ThyagaRajan & Priyanka, 2012).
Infantile microbiota colonisation is crucial in determining the adult microbiome health (Houghteling and Walker, 2015). External factors, such as detrimental changes to the mother’s diet or the development of microbial infections during pregnancy, may result in dysbiosis and abnormal foetal development that affects long-term health (Taddei et al., 2018; Taniya et al., 2022). Infants delivered vaginally (initially exposed to the vaginal bacteria of the mother) show a healthier bacteria composition compared to infants delivered via C-section (colonised by microbiota on the mother’s skin and in the surrounding environment) (Rosenfeld, 2015). C-section infants generally have a higher abundance of pathogenic microbes, some of which are attributed to the pathogenesis of Autism Spectrum Disorder (ASD), such as Clostridium spp. (Pandey et al., 2012; Penders et al., 2005, 2006).
The gut-microbiota-brain axis refers to the bidirectional communication of the enteric nervous system (ENS) in the gut and CNS (Goyal & Hirano, 1996). There are 3 main mechanisms by which the gut microbiota communicates information from the gut to the brain. Gut bacteria interact directly with immune cells in the gut, which make up 70-80% of all immune cells in the body (Vighi et al., 2008). Products of pathogenic bacteria bind to receptors on immune cells such as Toll-like receptors, triggering cytokine production (Fung et al., 2017). This also occurs when pathogenic metabolites from the gut microbiota enter systemic circulation and activate innate immunity (Borre et al., 2014). Pro-inflammatory cytokines can permeate the Blood-Brain Barrier and increase microglial activation, which in turn produces more pro-inflammatory cytokines. Thus, intestinal inflammation is closely related to neuroinflammation and subsequent neurodevelopment. Gut bacteria and its products are also able to interact directly with the vagus nerve and activate chemoreceptors in the nerve, affecting CNS functioning (Raybould, 2010). Additionally, the vagus nerve interacts with the peripheral immune system by being electrically stimulated when immune problems occur, and may up-regulate brain anti-inflammatory pathways (Meneses et al., 2016).
Studies showing the effects of the gut microbiome on brain development are corroborated by differences in microglial structure and functions between specific pathogen-free (SPF) mice and germ-free (GF) mice. Microglia contribute to proper neuronal connection and circuit regulation (Marín-Teva et al., 2004; Yamaguchi & Miura, 2015), and also participate in the formation/elimination of synapses. Studies suggest that they enhance dendritic spine density (Miyamoto et al., 2016). Erny et al. (2015) found that dendrite length, the number of branch points, and cell volume observed in the microglia of GF mice were all increased compared to that of SPF mice. Microglial cells from GF mice showed a more immature profile, increasing susceptibility to brain infections. Short-chain fatty acids (SCFAs), a bacterial product, influence microglia homeostasis. For example, Clostridia produces propionate SCFAs, which has been shown to increase microglial cell activation and production of pro-inflammatory cytokines, leading to ASD-like behaviours in mice (Choi et al., 2018; Silva et al., 2020).
In the context of gut dysbiosis, the predominance of pathobionts can lead to dysfunctional communication in the gut-brain axis. Microglia may respond abnormally to the enteric microbial stimuli, possibly causing neurodevelopmental disorders (NDDs), such as ASD (Cryan & Dinan, 2012).
ASD is a neurodevelopmental disorder that is diagnosed in approximately 1 in 100 children worldwide (Zeidan et al., 2022). The diagnostic criteria of ASD is characterised by a lack of social communication skills and repetitive behavioural patterns or interests (American Psychiatric Association, 2013), although everyday symptoms and comorbid disorders may be more wide-ranging. Post-mortem ASD brain tissue analysis revealed microglial aberrations, including increased microglial density and higher levels of cytokines (Gupta et al., 2014; Lee et al., 2017; Morgan et al., 2010). Microglial dysfunction may lead to increased dendritic spine density and excitatory synapses observed in ASD individuals (Martínez-Cerdeño, 2017)
Notably, gastrointestinal dysfunctions are the main commorbities developed by ASD individuals (Al-Beltagi, 2021). Chaidez et al. (2014) found that GI problems have been observed in 23% - 70% of patients with ASD, and some studies suggest reduced diversity in the microbiome of ASD compared with neurotypical (NT) individuals, with as a higher prevalence of Clostridium, Lactobacillus, and SCFAs found in ASD children (De Angelis et al., 2013; Wang et al., 2012). Some strains of Clostridium have been studied in ASD individuals; tetanus neurotoxins produced by C. tetani have been linked to an increase of ASD-like behaviours, while the development of GI issues may be attributed to exposure to C. bolteae (Li et al., 2019; Rosenfeld, 2015). Thus, dysbiosis and subsequent microglial dysfunction is implicated in the pathogenesis of ASD.
In order to evaluate if the gut microbiota could contribute to the development and symptoms of ASD, some forms of biological treatment based on antibiotics for mitigating ASD symptoms have been previously established. Although vancomycin was initially successful at reducing numbers of Clostridium and ASD symptoms in children, it did not selectively target bacteria, and affected other species of favourable bacteria during treatment (Bolte, 1998; Taniya et al., 2022). The beneficial effects of vancomycin treatment for ASD could only be observed up to two weeks after treatment completion, suggesting the need to implement more effective treatment techniques (Kang et al., 2019).
Some studies have used faecal microbiota transplantation (FMT) to modify gut microbiota of ASD individuals and improve ASD symptoms. FMT administered to 18 children with ASD by Kang et al. (2019) after vancomycin treatment and bowel cleanses revealed a long-term benefit to the gut microbiota and the lessening of symptoms. Health and behaviour status of participants was re-evaluated two years after the original treatment, showing a 58% average reduction of GI symptoms in the Gastrointestinal Symptom Rating Scale and a 47% decrease in ‘severity’ of ASD symptoms on the Childhood Autism Rating Scale (Kang et al. 2019). The reduction of symptoms may be linked to the healthy increase of faecal microbiota diversity in the participants two years after the original trial as well, given the low gut bacterial diversity in ASD.
Based on the studies suggesting that the modulation of the gut microbiota can influence ASD symptoms, a pilot study was conducted by Dr Thomas Louie (M Davoli-Ferreira, personal communication, October 12, 2023) at the University of Calgary, in which 10 ASD individuals underwent fecal microbiota transplantation (FMT), with 7 individuals showing a notable regression of clinical ASD symptoms. Analysis of fecal samples from the 10 ASD individuals both before and after FMT showed a loss of C. innocuum strains and reduction of 17 types of bacteria after treatment. As such, C. innocuum may be a pathobiont contributing to the development of ASD.
This project aims to determine the mechanisms by which C. innocuum strains isolated from ASD individuals may impact social behaviour, microglial numbers, and microglial morphology in a gnotobiotic mouse model.
Variables
Two strains of C. innocuum (Ci-8, Ci-7), isolated from ASD and neurotypical (NT) individuals, respectively, were monocolonised in the BTBR mouse model, with Germ-Free (GF) mice as the control group. The BTBR mouse model is an idiopathic model of autism. The monocolonisation of C. innocuum allowed for greater accuracy in determining its specific effects on the mouse.
The sociability and microglia morphology of each group of mice were analysed using specialised software (ANY-maze and IMARIS). Reduced sociability indicated increased ASD behavioural symptoms. Microglial and process density, process length, and soma size were analysed.
BTBR mice were used for all test groups; housing conditions and diet were identical for all mice. Mice were of similar ages (8-12 weeks) and were also sex-separated to identify any possible differences in the effects of C. innocuum in male and female mice. Procedures used to obtain brain tissue for immunofluorescence were kept constant for all mice.
Possible confounding variables included pre-existing conditions in the testing isolators (e.g. residual chemical odour in one area of the isolator) and differences in the effects of handling on each mouse, which may have distorted the accuracy of results. To avoid subjectivity, the experimenter performed a blinded analysis.
Procedure
General Animal Care
The BTBR T+Itpr3tf/J (BTBR) mouse model was used in this study (Meyza and Blanchard, 2017). This model displays the core behavioural deficits observed in autism, namely repetitive behaviours (McFarlane et al., 2008), reduced impulse control, reduced social and communication skills, and reduced accuracy in the detection of short stimuli (McTighe et al., 2013). Age-matched mice were randomly assigned to a neurotypical-derived (Ci-7) or an ASD-derived (Ci-8) C. innocuum strain and observed from 8-12 weeks of age. A minimum of 13 mice were used in each experimental group. A control group consisting of Germ-Free (GF) mice was also used. All mice were bred at the International Microbiome Centre (IMC) at the University of Calgary, and culture-dependent and culture-independent methods were employed to monitor GF status of mice. Mice were sex-matched; female and male mice were tested. Each cage housed 3-5 mice, with ad libitum access to both food and water. Autoclaved diets of starch and fish meal were provided.
Sociability Tests
The Three-Chamber test (Moy et al., 2004) was used to assess sociability of mice from each experimental group. In the sociability test, a GF mouse (‘stranger’) from the control group was placed in a wire cage in the left chamber, while the wire cage in the right chamber was empty. The mouse subject, from either experimental group, was initially placed in the middle chamber, with equal access to both the left and right chambers. The mice were able to smell, hear, and see each other, but no direct body contact could be made. The time (seconds) the mouse spent interacting with the empty wire cage and the wire cage with the stranger quantified the sociability of the mouse. Interaction was defined by the length of time the mouse spent making physical contact with (e.g. pawing or sniffing) the wire cages, not the time the mouse spent in each chamber in general.
A Social Novelty (SN) test was also performed using the three-chambered apparatus. A new stranger GF mouse was placed in the empty wire cage on the right, and the old stranger remained in the wire cage on the left. The mouse subject was, again, initially placed in the middle chamber. The parameters of ‘interaction’ for the SN test were the same as that described for the sociability test. Therefore, increased interaction with the new stranger represented a greater social preference for novel mice than familiar mice. Using the video-tracking system ANY-maze (Stoelting Co., 2023), the movement of the mouse subject in the apparatus was recorded over a length of 10 minutes for each test. Each mouse in each experimental group underwent one sociability test and one social novelty test.
After all the behaviour tests were performed, the mice were sacrificed for brain tissue analysis.
Immunofluorescence and Microglia Analysis
Immunofluorescence microscopy was performed on the brain slices. The mice were deeply anaesthetised and perfused transcardially with 0.9% saline solution, followed by fresh 4% paraformaldehyde (PFA) in 0.1-M phosphate buffer (pH 7.4). After the perfusion, the brains were dissected and post-fixed for 24hr in PFA and then replaced with 30% sucrose overnight. Brain sections (free-floating in PBS, 40 μm), embedded in Tissue-Tek O.C.T. Compound, were cut in a Leica CM1860 UV cryostat (Leica Biosystems, Wetzlar, Germany) at -19 to -21ºC. The floating sections were used for immunofluorescence assays.
The sections were transferred to a blocking buffer (0.3% Triton X-100 and 1-2% BSA in PBS), and incubated for 1hr at room temperature on an orbital shaker, then incubated overnight at 4°C with the polyclonal primary antibody for IBA-1 (1:1000; Wako Chemicals, Richmond, VA), a marker for microglial cells. After washing, the sections were incubated with the secondary antibody solution for 1hr at room temperature (1:500; IgG-conjugated Alexa Fluor 555). The sections were washed with PBS and mounted on glass slides, then covered with cover slips with Fluoromount Aqueous Mounting Medium (Sigma-Aldrich, Massachusetts, United States). Images were acquired using a confocal laser-scanning microscope. 3D visualisations of the microglia in the cortex of the brains were created using Microscopy Image Analysis Software (IMARIS; Andor, 2024); length, density, and ramification of dendritic processes were traced. The numbers of microglial cells in the brains were also counted.
Data taken from ANY-maze and IMARIS analysis was used to identify behavioural and neuro-morphological differences between Ci-7 (NT) and Ci-8 (ASD) mice.
Observations
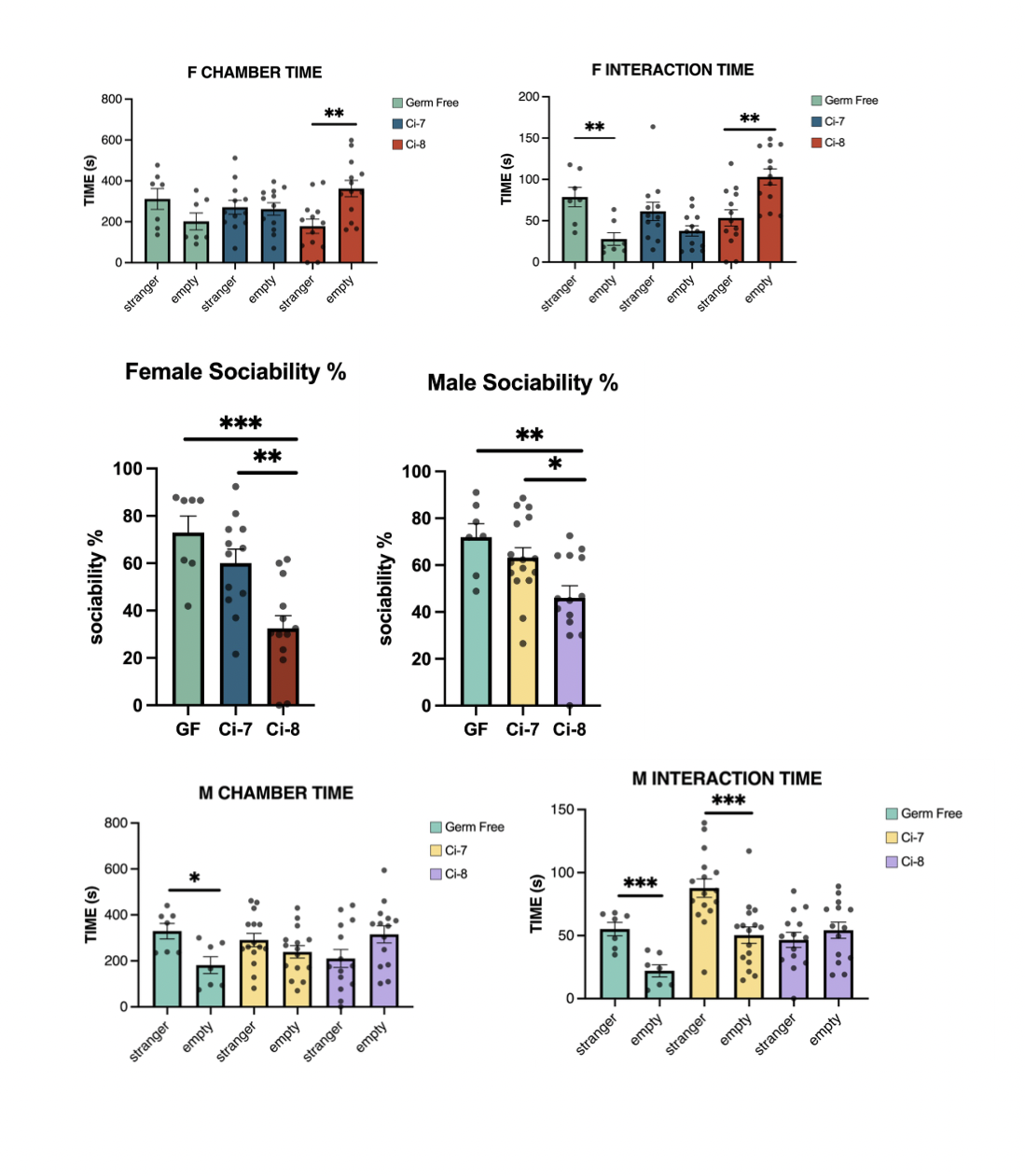
Female BTBR mice monocolonised with Ci-8 showed significantly increased interaction with the empty cage than the stranger cage in the Three-Chamber Sociability Test, and significantly lower sociability percentage, while those monocolonised with Ci-7 did not have significant differences in interaction time. Male BTBR mice monocolonised with Ci-8 did not have significant differences in interaction time, while those monocolonised with Ci-7 showed significantly increased interaction with the stranger cage than the empty cage.
There were no significant differences in interaction times with the familiar and novel strangers or in social novelty preference percentage in either Ci-7 or Ci-8 BTBR mice, as well as the control group.
Significant differences in interaction times were determined by an unpaired t test, *p≤0.05, **p≤0.01, ***p≤0.001; ns=p>0.05. Sociability percentage was calculated for males and females based on time investigating social object (mouse) divided by total time investigating both social and inanimate objects (empty cage). Significant differences in sociability percentage were determined by a one-way ANOVA with post-hoc Tukey’s test, *p≤0.05, **p≤0.01, ***p≤0.001; ns=p>0.05.
Analysis
Both male and female Ci-8 mice displayed lower sociability than Ci-7 mice, indicating that the ASD-derived strain affects development of BTBR mice such that it alters some social behaviours. However, the lack of social novelty preference of the mice indicates that Ci-8 only modulates specific social behaviours. Previous studies with mouse models of autism also showed an absence of social novelty preference (Carter, 2011); the pathogenesis of ASD may not affect social novelty behaviour.
Conclusion
ASD-derived C. innocuum (Ci-8) promotes reduced sociability in BTBR mice and does not alter social novelty preference in BTBR mice. As such, Ci-8 may only modulate specific social behaviours.
Application
Analysis of microglial morphology of the mice is in progress. It is hypothesised that mice monocolonised with ASD-derived C. innocuum will display increased microglial density, soma size, and process density and length, as abnormal microglia ramification has been correlated with ASD.
The average prevalence of ASD worldwide has been estimated to be 0.6% (Salari et al., 2022), with an increasing annual prevalence, although this has been partly attributed to a more inclusive understanding of the types of ASD symptoms and the ways that they can be manifested in individuals, as well as an increase in ASD awareness as an NDD (Nevison & Blaxill, 2017). ASD is a matter of public concern due to its early age of onset and associated impairments (Simonoff et al., 2008). Microbial-based interventions for ASD have only been explored in recent years; vancomycin treatment is not effective in the long term (Bolte, 1998) and probiotic treatments have not been reliably tested for long term effects (Kang et al., 2017). Although FMT has been shown to be more successful in improving ASD symptoms long term (Kang et al., 2019), additional research should be conducted on microbes’ effects on ASD development to gain a more stable footing in understanding the role that the microbiome plays in ASD. Furthermore, a greater understanding could lead to future developments in effective microbial-based interventions for ASD, thereby making potential treatment options more accessible for ASD individuals. Increased numbers of Clostridium spp. have been found in ASD compared to NT individuals, indicating a connection to ASD.
This study enhances understanding of C. innocuum’s effect on ASD development. Additionally, males have a higher rate of ASD diagnosis compared to females, with the ratio of diagnosed males to females being 3:1 (Loomes et al., 2017). Therefore, it is also useful for the study to differentiate between male and female test groups in order to identify whether C. innocuum is likely to have an increased effect on one sex over the other in mice.
Sources Of Error
A larger sample size of n ≥ 18 may result in data for the sociability tests that is more representative of the effects of C. innocuum. The lack of significant differences in Social Novelty test results may be due to the age of the subject mice. Because early-life alterations in microglia and neuronal morphology is implicated in the pathogenesis of ASD, a younger age range of mice tested may show differences in social novelty preference. Only adult mice (8-12 weeks of age) underwent the sociability tests; external factors may have altered the social behaviour of the mice as they matured. However, very young mice do not yield reliable results in sociability testing, as they do not display adequate social preferences until 6 weeks of age. Testing alternate behavioural phenotypes in younger mice, such as ultrasonic vocalisations (USV), may provide additional insight into the role of C. innocuum in early development. However, due to the germ-free conditions of the International Microbiome Centre, where testing is conducted, materials must be autoclaved before entering the Centre; USV detectors cannot be autoclaved without suffering damage.
Citations
Al-Beltagi, M. (2021). Autism medical comorbidities. World Journal of Clinical Pediatrics, 10(3), 15–28. https://doi.org/10.5409/wjcp.v10.i3.15
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/10.1176/appi.books.9780890425596
Andor Technology Ltd. (2021). Interactive Microscopy Image Analysis software (Version 9.8.1) [Computer software]. https://imaris.oxinst.com
Bolte, E. R. (1998). Autism and Clostridium tetani. Medical Hypotheses, 51(2), 133–144. https://doi.org/10.1016/s0306-9877(98)90107-4
Borre, Y. E., O’Keeffe, G. W., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2014). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends in Molecular Medicine, 20(9), 509–518. https://doi.org/10.1016/j.molmed.2014.05.002
Carter, M. D., Shah, C. R., Muller, C. L., Crawley, J. N., Carneiro, A. M. D., & Veenstra-VanderWeele, J. (2011). Absence of preference for social novelty and increased grooming in integrin β3 knockout mice: initial studies and future directions. Autism Research: Official Journal of the International Society for Autism Research, 4(1), 57–67. https://doi.org/10.1002/aur.180
Chaidez, V., Hansen, R. L., & Hertz-Picciotto, I. (2014). Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders, 44(5), 1117–1127. https://doi.org/10.1007/s10803-013-1973-x
Choi, J., Lee, S., Won, J., Jin, Y., Hong, Y., Hur, T.-Y., Kim, J.-H., Lee, S.-R., & Hong, Y. (2018). Pathophysiological and neurobehavioral characteristics of a propionic acid-mediated autism-like rat model. PloS One, 13(2), e0192925. https://doi.org/10.1371/journal.pone.0192925
Cryan, J. F., & Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews. Neuroscience, 13(10), 701–712. https://doi.org/10.1038/nrn3346
De Angelis, M., Piccolo, M., Vannini, L., Siragusa, S., De Giacomo, A., Serrazzanetti, D. I., Cristofori, F., Guerzoni, M. E., Gobbetti, M., & Francavilla, R. (2013). Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PloS One, 8(10), e76993. https://doi.org/10.1371/journal.pone.0076993
Erny, D., Hrabě de Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., Keren-Shaul, H., Mahlakoiv, T., Jakobshagen, K., Buch, T., Schwierzeck, V., Utermöhlen, O., Chun, E., Garrett, W. S., McCoy, K. D., Diefenbach, A., Staeheli, P., Stecher, B., Amit, I., & Prinz, M. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience, 18(7), 965–977. https://doi.org/10.1038/nn.4030
Fung, T. C., Olson, C. A., & Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nature Neuroscience, 20(2), 145–155. https://doi.org/10.1038/nn.4476
Goyal, R. K., & Hirano, I. (1996). The enteric nervous system. The New England Journal of Medicine, 334(17), 1106–1115. https://doi.org/10.1056/NEJM199604253341707
Gupta, S., Ellis, S. E., Ashar, F. N., Moes, A., Bader, J. S., Zhan, J., West, A. B., & Arking, D. E. (2014). Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nature Communications, 5, 5748. https://doi.org/10.1038/ncomms6748
Houghteling, P. D., & Walker, W. A. (2015). Why is initial bacterial colonisation of the intestine important to infants’ and children's health? Journal of Pediatric Gastroenterology and Nutrition, 60(3), 294–307. https://doi.org/10.1097/MPG.0000000000000597
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., & Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World Journal of Gastroenterology: WJG, 21(29), 8787–8803. https://doi.org/10.3748/wjg.v21.i29.8787
Kang, D.-W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., Khoruts, A., Geis, E., Maldonado, J., McDonough-Means, S., Pollard, E. L., Roux, S., Sadowsky, M. J., Lipson, K. S., Sullivan, M. B., Caporaso, J. G., & Krajmalnik-Brown, R. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome, 5(1), 10. https://doi.org/10.1186/s40168-016-0225-7
Kang, D.-W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., Caporaso, J. G., & Krajmalnik-Brown, R. (2019). Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Scientific Reports, 9(1), 5821. https://doi.org/10.1038/s41598-019-42183-0
Lee, A. S., Azmitia, E. C., & Whitaker-Azmitia, P. M. (2017). Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain, Behavior, and Immunity, 62, 193–202. https://doi.org/10.1016/j.bbi.2017.01.019
Li, N., Yang, J., Zhang, J., Liang, C., Wang, Y., Chen, B., Zhao, C., Wang, J., Zhang, G., Zhao, D., Liu, Y., Zhang, L., Yang, J., Li, G., Gai, Z., Zhang, L., & Zhao, G. (2019). Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment. Genomics, Proteomics & Bioinformatics, 17(1), 26–38. https://doi.org/10.1016/j.gpb.2019.01.002
Loomes, R., Hull, L., & Mandy, W. P. L. (2017). What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(6), 466–474. https://doi.org/10.1016/j.jaac.2017.03.013
Marín-Teva, J. L., Dusart, I., Colin, C., Gervais, A., van Rooijen, N., & Mallat, M. (2004). Microglia promote the death of developing Purkinje cells. Neuron, 41(4), 535–547. https://doi.org/10.1016/s0896-6273(04)00069-8
Martínez-Cerdeño, V. (2017). Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Developmental Neurobiology, 77(4), 393–404. https://doi.org/10.1002/dneu.22417
McFarlane, H. G., Kusek, G. K., Yang, M., Phoenix, J. L., Bolivar, V. J., & Crawley, J. N. (2008). Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain, and Behavior, 7(2), 152–163. https://doi.org/10.1111/j.1601-183X.2007.00330.x
McTighe, S. M., Neal, S. J., Lin, Q., Hughes, Z. A., & Smith, D. G. (2013). The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PloS One, 8(4), e62189. https://doi.org/10.1371/journal.pone.0062189
Meneses, G., Bautista, M., Florentino, A., Díaz, G., Acero, G., Besedovsky, H., Meneses, D., Fleury, A., Del Rey, A., Gevorkian, G., Fragoso, G., & Sciutto, E. (2016). Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. Journal of Inflammation , 13, 33. https://doi.org/10.1186/s12950-016-0140-5
Meyza, K. Z., & Blanchard, D. C. (2017). The BTBR mouse model of idiopathic autism - Current view on mechanisms. Neuroscience and Biobehavioral Reviews, 76(Pt A), 99–110. https://doi.org/10.1016/j.neubiorev.2016.12.037
Miyamoto, A., Wake, H., Ishikawa, A. W., Eto, K., Shibata, K., Murakoshi, H., Koizumi, S., Moorhouse, A. J., Yoshimura, Y., & Nabekura, J. (2016). Microglia contact induces synapse formation in developing somatosensory cortex. Nature Communications, 7, 12540. https://doi.org/10.1038/ncomms12540
Morgan, J. T., Chana, G., Pardo, C. A., Achim, C., Semendeferi, K., Buckwalter, J., Courchesne, E., & Everall, I. P. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological Psychiatry, 68(4), 368–376. https://doi.org/10.1016/j.biopsych.2010.05.024
Moy, S. S., Nadler, J. J., Perez, A., Barbaro, R. P., Johns, J. M., Magnuson, T. R., Piven, J., & Crawley, J. N. (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain, and Behavior, 3(5), 287–302. https://doi.org/10.1111/j.1601-1848.2004.00076.x
Nevison, C. D., & Blaxill, M. (2017). Diagnostic Substitution for Intellectual Disability: A Flawed Explanation for the Rise in Autism. Journal of Autism and Developmental Disorders, 47(9), 2733–2742. https://doi.org/10.1007/s10803-017-3187-0
Pandey, P. K., Verma, P., Kumar, H., Bavdekar, A., Patole, M. S., & Shouche, Y. S. (2012). Comparative analysis of fecal microflora of healthy full-term Indian infants born with different methods of delivery (vaginal vs cesarean): Acinetobacter sp. prevalence in vaginally born infants. Journal of Biosciences, 37(6), 989–998. https://doi.org/10.1007/s12038-012-9268-5
Penders, J., Thijs, C., Vink, C., Stelma, F. F., Snijders, B., Kummeling, I., van den Brandt, P. A., & Stobberingh, E. E. (2006). Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics, 118(2), 511–521. https://doi.org/10.1542/peds.2005-2824
Penders, J., Vink, C., Driessen, C., London, N., Thijs, C., & Stobberingh, E. E. (2005). Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiology Letters, 243(1), 141–147. https://doi.org/10.1016/j.femsle.2004.11.052
Raybould, H. E. (2010). Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Autonomic Neuroscience: Basic & Clinical, 153(1-2), 41–46. https://doi.org/10.1016/j.autneu.2009.07.007
Salari, N., Rasoulpoor, S., Rasoulpoor, S., Shohaimi, S., Jafarpour, S., Abdoli, N., Khaledi-Paveh, B., & Mohammadi, M. (2022). The global prevalence of autism spectrum disorder: a comprehensive systematic review and meta-analysis. Italian Journal of Pediatrics, 48(1), 112. https://doi.org/10.1186/s13052-022-01310-w
Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47(8), 921–929. https://doi.org/10.1097/CHI.0b013e318179964f
Stoelting Co.. (2023). ANY-maze Video Tracking Software (Version 7.36) [Computer software]. https://www.any-maze.com
Acknowledgement
Dr. Kathy McCoy, University of Calgary, Department of Physiology and Pharmacology
Dr. Marcela Davoli-Ferreira, Cumming School of Medicine, University of Calgary
Dr. Garcia-Diaz, Webber Academy Science Coordinator