The Greatest Battle of the Century 'pH Vs. Boiling point'
Grade 5
Presentation
Hypothesis
If water is boiled while having a acidic or alkaline pH then it will boil at the normal temperature (that being ~100°C) because the hydroxide and hydrogen ions don't affect the vapor or atmospheric pressure and those 2 things affect the boiling point sinse water boils when the vapor pressure and atmospheric pressure become equal.
Research
1. Q: How does water boil?
A: Water boils when the vapor pressure and the atmospheric pressure become equal then the water boils
2. Q: Do acidic things affect the water at all or not?
A: An acidic environment can change the pH of the water by making it more acidic
3. Q: What variables affect the boiling point of water?
A: The only variables that affect the boiling point of water are the vapor pressure and the atmospheric pressure
4. Q: What affects vapor pressure?
A: The only variable that affects the vapor pressure is the temperature of the water.
5. Q: Does pH affect the water molecules?
A: pH isn't anything with the water molecules but they are the amount of hydrogen and hydroxide ions
Variables
Responding variable
The temperature the water boils at
Manipulated variable
The pH
Controlled variables
1. Same kettle
2. Same rate of heating
3. Same thermometer
4. Same starting temperature
Procedure
- Put all the cups of water on the same counter for 1 hour until they are all the same temperature
- Put a 1tsp of baking soda in one cup of water and mix it then put 2tsp of baking soda in another cup of water and mix it
- Put 1tsp of soap water in one cup of water and mix it then put 2 tsp soap water in another cup of water and mix it
- Put 1tsp of lemon juice in one cup of water and mix it then put 2tsp of lemon juice in another cup of water and mix it
- Put 1 tsp of vinegar in one cup of water and mix it then put 2tsp of vinegar in another cup of water and mix it
- Put 1 cup of plain water in one cup
- See all the different pH using a pH strip and record it in a chart
- Pour the plain water into the kettle
- Put a thermometer in the kettle
- Turn on kettle
- Wait until it boils and record the temperature at the water begins to boil with an adult supervisor
- Repeat for each pH 3 times and record results in the chart
Materials
- Kettle/ Container
- Baking Soda
- Water
- Lemon Juice
- Vinagar
- Soap
- pH slips
- Thermometer
Observations
|
Control (pH 7) |
2 Tsp Vinegar (pH 4) |
1Tsp Vinegar (pH 4) | 1Tsp Lemon Juice (pH 4) | 2 Tsp Lemon Juice (pH3) | 1Tsp Soap/ Detergent (pH 7.5) | 2Tsp Soap/ Detergent (pH 8) | 1Tsp Baking Soda (pH 8) | 2 Tsp Baking Soda (pH 8) | |
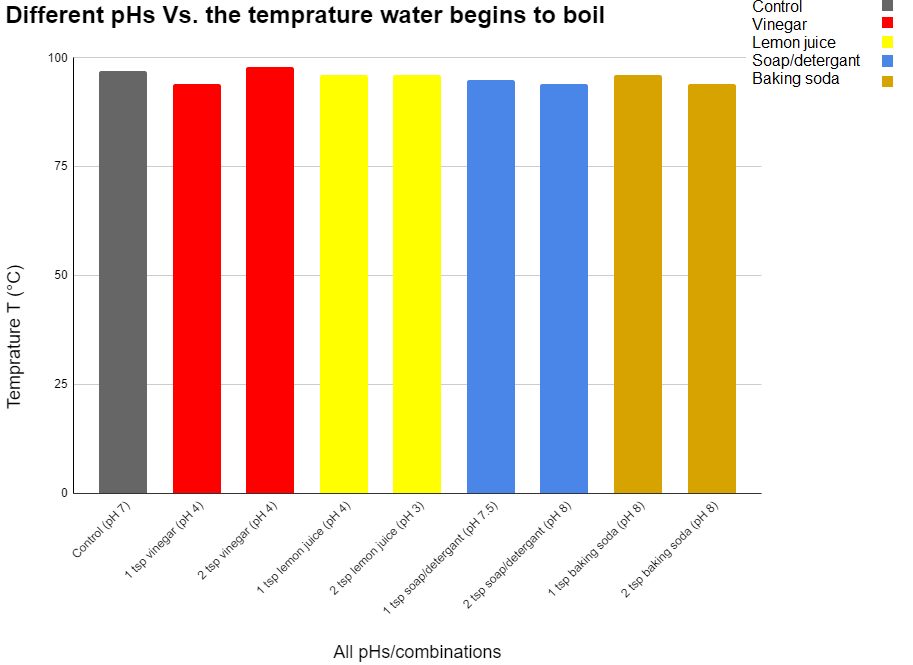
| Trial 1 | 97°C | 96°C | 95.6°C | 96.8°C | 96.7°C | 94.3°C | 93.6°C | 95.7°C | 95°C |
| Trial 2 | 96.3°C | 99°C | 94°C | 95.5°C | 96°C |
96°C |
94°C | 96.5°C | 93.5°C |
| Trial 3 | 98.5°C | 98°C | 92°C | 96.7°C | 96.5°C | 96°C | 95°C | 96.6°C | 94°C |
Connection to Research
All of the results were between 92-99°C. It seems that the pH didn’t affect the boiling point sinse all of the pHs asidic and basic were near the same range. Like I saw in the research the only thing that affects vapor pressure is temperature (atmospheric pressure is altitude). Vapor and amtospheric pressure are the only 2 things that affect the boiling point because when the 2 pressures become equal water boils. So because the only thing that affects atmospheric pressure is altitude and the only thing that affects vapor pressure is temperature. The pH doesn’t affect temperature it didn’t affect the boiling point.
Soap Water Paragraph
The water with soap inside of it would sometimes foam up so much you couldn’t see anything inside the water. Aswell it took a long time to boil that eventually we had to put a lid on the water while it was warming up so we could speed up the boiling.
Lemon Juice Paragraph
The lemon juice was like the soap the soap but less foam and a different colour. It looked like normal water but it foamed up and smelled sour.
Vinegar Water Paragraph
Since I used apple cider vinegar the water vapor smelled very strong (and bad). Also the water with 1 tsp of vinegar boiled pretty low temperatures (considering the other tests) and it was the same pH of the 2tsp so it had to be something else. It boiled slowly and I had to be patient until it was done.
Baking Soda Water Paragraph
The water would only foam on the edes of the kettle. Aswell it would bubble alot on the bottom of the kettle and it boiled very quickly.
Analysis
Averages
Control = 97°C
2 Tsp Vinegar = 98°C
1 Tsp Vinegar = 94°C
1 Tsp Lemon Juice = 96°C
2 Tsp Lemon Juice = 96°C
1 Tsp Soap/ Detergent = 95°C
2 Tsp Soap / Detergent = 94°C
1 Tsp Baking Soda = 96°C
2 Tsp Baking Soda = 94°C
Conclusion
The pH did not affect the boiling point because all of the mixtures were around the same temperature when beginning to boil but because the temperature was still different it is the materials themselves that chaged the vapor pressure in some way but it was not the pH.
Application
I think people would want to know this because lets say you put some alkaline or asidic sweetners or somthing in your tea. You might think "Wait I know when norma tea boils but what about alkaline or asidic tea what if it takes too long?". But after this project I can tell people not to worry for the example person's tea is going to start boiling at it's normal boiling point so they don't have to worry! Aswell I would make my project better another time by tring to have more varity of pH so I could test more types and maybe get different info. That's the main thing but some small things are me somtimes measuring the temperature too early or even too late next time I want to work on having more of a time I think it is boiling.
Sources Of Error
I had mistakingly not followed one of my controled variables (same kettle) by using a can to boil the soap water instead of the kettle. Aswell I didn't have an excact point I called boiling and thet would change the results a bit because of my bias.
Citations
"Boiling" edited RN. Jacobson, Wikipedia, Wikimedia Foundation, 19 Nov. 2004, en.wikipedia.org/wiki/boiling
Fondriest Environmental, Inc."pH of water" Fundamentals of Environmental Measurements 19 Nov. 2013. Web.<https://www.measurments/parameters/water-quality/ph/>
"pH and Water"USGS Oct.22, 2019 Online
"vapour pressure" Libre Texts Jan. 30 2023 Online
Acknowledgement
My mom - for helping me with digital stuff and helping me understand topics, supporting me in general and helping me finish
Ablah (my sister) - for looking at my work and approving and helping me make it better
My sibilings - for helping me and letting me go on the computer so I can work on it
Mr. Knitter - for talking to me about these topics and making me understand them more