Development of mRNA cancer vaccines to cure Pancreatic Ductal Adenocarcinoma
Grade 10
Presentation
Problem
How can personalized mRNA neoantigen vaccines treat patients with Pancreatic Ductal Adenocarcinoma?
- What is the function and parts of the pancreas, and where does Pancreatic Ductal Adenocarcinoma occur?
- Why are traditional forms of immunotherapy such as immune checkpoint inhibitors ineffective against Pancreatic Ductal Adenocarcinoma?
- How do neoantigens stimulate the immune system of patients with Pancreatic Ductal Adenocarcinoma?
Method
- Firstly, I will be using various online resources including videos, websites, and news articles to research and present about Pancreatic Ductal Adenocarcinoma.
- The next part of my research will be about the inefficacy of traditional forms of immunotherapy, specifically immune checkpoint inhibitors, on PDAC. I will then research how mRNA cancer vaccines work to treat PDAC.
- To support my research, I will be referring to specific data collected from a phase 1 clinical trial conducted to test mRNA vaccines against Pancreatic Ductal Adenocarcinoma.
Research
- What is the pancreas? The pancreas is a gland that is located in the abdomen. It plays a key role in converting the food that we consume into fuel. It has two functions: an exocrine function carried out by the exocrine glands that helps in digestion and an endocrine function carried out by endocrine glands that regulates blood sugar. The exocrine pancreas cells help the gland to make juices which help digest food, and the endocrine pancreas cells help to make hormones which control blood sugar levels. 95% of pancreatic cancers begin are exocrine tumors and are known as adenocarcinomas.
- Location of the pancreas: The pancreas lies between the stomach and the spine in the upper left abdomen. It is surrounded by other organs including the small intestine, liver, and spleen. The pancreas is shaped like a flat-horizontal pear, with a wide part that tapers down to a narrow part. The wide part is known as the head, the middle part is the neck or the body and the thin end extends to the left side and is known as the tail. The pancreas is surrounded by several major blood vessels which supply blood to the pancreas and other abdominal organs/glands.
- Functions of the pancreas: The pancreas have two main functions, the exocrine and endocrine functions:
- Function of exocrine glands: The exocrine pancreatic glands produce natural juices known as pancreatic enzymes. These enzymes include chymotrypsin and trypsin to digest proteins, amylase for the digestion of carbohydrates, and lipase to break down and digest fats. When the pancreas needs to digest food, these pancreatic juices go through a series of ducts and accumulate in the pancreatic duct. This pancreatic duct connects to the common bile duct which starts out from the liver, and forms the ampulla of Vater in a part of the small intestine known as the duodenum. The pancreatic juices from the pancreatic duct and the bile from the common bile duct are released into the duodenum where fats, carbohydrates, and proteins are digested.
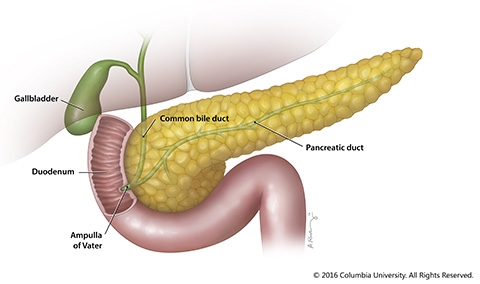
- Function of endocrine glands: The endocrine glands of the pancreas contain a type of specialized cells known as islet cells. These cells are responsible for secreting hormones into the bloodstream. The main hormones that the endocrine component of the pancreas releases is the insulin, which helps to lower blood sugar levels, and glucagon, which helps to increase blood sugar levels.
Pancreatic Tumors:
- Normally, cells only divide to form new cells when the body needs new cells. However, mutations in the DNA of cancerous cells cause them to divide and reproduce much faster and rapidly than normal cells. This quick reproduction of unhealthy cancerous cells outcompetes the growth of normal cells and eventually forms a tumor. Tumors can be either benign or malignant. Benign tumors usually stay in one place where they originated from and do not spread to other parts of the body, so they are not considered cancerous. On the other hand, malignant tumors can start invading other parts of the body such as major organs or tissues, so these are considered cancerous.
- Exocrine tumors and pancreatic ductal adenocarcinoma (PDAC): 95% of pancreatic cancers develop in the exocrine pancreatic glands. Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer. It is caused by cancerous cells lining the pancreatic duct. PDAC occurs in the head of the pancreas. The growth of cancerous tumors in the head of the pancreas can compress the bile duct which can result in obstructive jaundice when bile flow out of the liver is blocked. PDAC can metastasize early to the livers, the peritoneum, the lungs, and the bones.
- Pancreatic cancer has a high mortality rate and is deadly in 88% of the people who are diagnosed with it. The ability of the pancreatic tumor to metastasize rapidly causes it to spread to different body parts. Oftentimes, even before diagnosis, the tumor would have already metastasized. This way, even if the tumor is removed surgically, it is difficult to rid the entire body of the cancer. As well, almost 80% of the patients who undergo surgery for PDAC do not have a recurrence free survival and end up relapsing. For patients with advanced PDAC, the most commonly used treatment in an aggressive form of chemotherapy known as FOLFIRINOX, but PDAC tumours are oftentimes chemotherapy-resistant.
Why are traditional forms of immunotherapy such as immune checkpoint inhibitors (ICIs) ineffective against Pancreatic Ductal Adenocarcinoma?
Immune checkpoint inhibitors against Pancreatic Ductal Adenocarcinoma (PDAC):
- Before the breakthrough of mRNA cancer vaccines, several clinical trials were conducted testing the effectiveness of a form of immunotherapy known as immune checkpoint inhibitors against PDAC. However, these failed to even stimulate a response in the immune system and did not prove effective against PDAC. How do immune checkpoint inhibitors work?
- An important type of immune cell in our bodies are T-cells. These cells protect us from infections and bacteria, viruses, and other harmful microorganisms. T cells have proteins called checkpoint proteins on their surface. These checkpoint proteins regulate the immunogenicity of the T-cells, and are responsible for switching off T-cells when required. In other words, these checkpoint proteins lessen the activity of T-cells, and are necessary for survival when present in moderate amounts.
- Cancer cells, however, have types of receptors which can increase the presence of these checkpoint proteins on T-cells. With the increasing presence of checkpoint proteins on T-cells, T-cells become less efficient and effective at recognizing, tracking down, and killing tumor cells.
- Immune checkpoint inhibitors are types of drugs which “inhibit” or stop the cancer receptors from increasing the presence of checkpoint proteins on T-cells. Therefore, these checkpoint inhibitors allow for the immune system to be turned back on, and fight the immunosuppressive nature of tumors.
- This image below illustrates this. PD-1 is a type of checkpoint protein on immune cells, and PD-L1 is an antigen on tumor cells. PD-L1 usually binds to PD-1 and suppresses the immune system. However, an anti PD-L1 blocker, which is an immune checkpoint inhibitor, stops this immunosuppressive effect.
Image Source
Reason for the ineffectiveness of immune checkpoint inhibitors in PDAC:
- PDAC tumors have a lower mutation burden compared to other cancers: In order for immune checkpoint inhibitors to be effective, they need to be able to bind to a specific mutation on the tumor. However, PDAC tumors have a much smaller mutation rate, or a mutational burden compared to other cancers. The smaller mutation results in the presence of a fewer number of tumor-specific antigens, and therefore a reduced sensitivity of PDAC tumors to checkpoint inhibitors. As well, the small number of tumor-specific antigens are not sufficiently represented to stimulate an immune response from T-cells which is a reason why checkpoint inhibitors are not effective against PDAC tumors.
- To overcome this obstacle of the lack of immunogenicity of checkpoint inhibitors, mRNA vaccines increase the presence of these tumor-specific antigens, known as neoantigens on the surface of immune cells. With this increase in the presence of these tumor-specific antigens, immune cells are able to recognize the PDAC tumors are foreign and therefore attack.
How do mRNA vaccines work:
The mRNA vaccine first encodes for the antigens present on the surface of a tumor. Once the vaccine is injected, cells use the mRNA as a template to create the tumor proteins. These foreign proteins trigger an immune response and cause the healthy cells in the pancreas to develop antibodies against the cancerous cells. When the cells with the antibodies are exposed to the tumors, the antibodies will recognize and attach to them. These antibodies prevent the tumors from spreading.
- Cancer immunotherapy works by strengthening the body’s immune response to invading and tumor cells.
- The human immune system can protect the body against foreign substances such as infections by using a type of white blood cells known as memory cells which have a memory of every microbe or pathogen that has invaded the body. This memory of the invading organism allows for the white blood cells to develop antibodies that can specifically bind to markers and proteins on invading cells known as antigens. When these antibodies can specifically bind to the antigens, the antibodies can successfully stop the growth of the invading organism or cells.
- However, the reason that our immune system is oftentimes ineffective against cancers is because all cancer cells are the mutations of the body’s own cells. This way, the immune system has trouble recognizing them as foreign and cannot successfully suppress the growth of cancer cells. As well, the surveillance capacity of the immune system is gradually weakened as the tumor/cancer cells grow more mutations. Tumor cells which are, in early stages of development, caught by the immune system, eventually are screened out as they develop mutations known as tumor molecules which are not recognized by the immune system as foreign.
- Several immunotherapy methods have been ineffective against Pancreatic Ductal Adenocarcinoma. A reason for this is because PDAC tumor cells have less mutations than the cancer cells of other cancers. This way, many man made antibodies known as monoclonal antibodies do not have enough antigens or proteins on tumor cells to bind to and suppress. However, recent studies have shown that patients with PDAC who have longer survival rates and a higher Relapse Free Survival (RFS) tend to harbor special types of proteins specific to tumor cells known as neoantigens.
- To overcome this problem, medical researchers have been looking to strengthen the immune system’s anti-tumor response. Current immunotherapies use types of antigens on tumor cells known as neoantigens.
- What are neoantigens: Neoantigens are tumor specific antigens which are the result of mutations in tumor cells. These are expressed solely on the surface of tumor cells, and are unique to each tumor cell. These are the basis of current immunotherapy methods, such as mRNA vaccines, as they act as ideal candidates for T-cells (a type of white blood cells) to bind to.
Image Source
mRNA cancer vaccines in patients with Pancreatic Ductal Adenocarcinoma:
- In patients with Pancreatic Ductal Adenocarcinoma, the tumor cells are surgically resected from the patient’s body. The DNA of these cancer cells are then scanned to locate the most mutated parts of the cell, known as the neoantigens. These neoantigens are then placed in between the messenger RNA or the mRNA. This mRNA then has the capability to replicate these tumor specific proteins (neoantigens) on the surface of cells. By allowing cells to replicate these neoantigens, the immune system, specifically the T-cells, are stimulated as they now have the information required to build the necessary antibodies against these tumor antigens, or the neoantigens.
- Recently, the pharmaceutical company BioNtech created personalized mRNA vaccines for a clinical study of 16 individuals with Pancreatic Ductal Adenocarcinoma. The reason for the requirement of a personalized vaccine is because each individual’s neoantigens on the surface of their tumor cells are slightly different, so each individual would require slightly different vaccines with slightly different mutations encoded in the mRNA.
Administration of the mRNA cancer vaccine for PDAC in the BioNtech trial:
-
The 16 patients who were enrolled in the trial had their PDAC tumors resected surgically.
- The procedure used to resect these tumors was the whipple procedure, or the pancreaticoduodenectomy. This is the primary surgical procedure used to treat pancreatic cancer and involves the removal of the head of the pancreas, the first part of the small intestine known as the duodenum, the gallbladder and the bile duct.
- A second surgical procedure known as distal pancreatectomy and splenectomy was performed to resect the body and the tail of the pancreas, as well as the spleen (splenectomy). The reason for the removal of the spleen is because in Pancreatic Ductal Adenocarcinoma, the tumor often invades the splenic artery or vein.
-
These tumors were then shipped within 72 hours to a manufacturing facility, where exome sequencing was performed on them.
- Exome sequencing: Sequences of DNA form genes. While some genes carry out instructions for cellular functions and mechanisms, many genes do not do this. Researchers estimate that only 1-2% of the human genome contains information that can be coded to form proteins and carry out functions. This coding region of the DNA is known as the exome. Since researchers have determined that around 85% of all genetic diseases are caused by mutations in the exome, whole exome sequencing allows them to efficiently identify which genes have mutations in them.
- Exome sequencing looks for changes in the sequence of nucleic bases (Adenine, Guanine, Thymine, Cytosine) that could result in mutations which would cause the development of proteins on mutated cells. Exome sequencing is a cost-effective and efficient way to scan DNA as it can simultaneously scan the various genes in the exome (protein coding region) of the DNA.
- In the pancreas of the patients with PDAC, exome sequencing was used as a way to scan the DNA of the cells to recognize the mutated regions of the tumor cells.
-
In the third step, the tumors underwent RNA sequencing.
- RNA sequencing (RNA seq) allows researchers to investigate and analyze the transcriptome which is the total content of the RNA including the mRNA (messenger RNA), rRNA (ribosomal RNA), and tRNA (transfer RNA). RNA seq shows which genes are turned on or off, or where there are certain changes which could result in the translation of a different type of protein.
- RNA sequencing is used to identify which parts of the RNA, specifically the mRNA, codes for the neoantigens which are present on the surface of tumor cells.
- Computational identification of the neoantigens allowed for the individualized/personalized mRNA vaccines to be manufactured incorporating the neoantigens specific for each patient enrolled in the trial.
-
During this manufacturing process of the mRNA vaccines, patients enrolled in the trial were given a targeted therapy drug known as atezolizumab, which is an anti PDL-1 blocking antibody.
- Atezolizumab is a monoclonal antibody (man made antibody) which binds to a protein present on many tumor cells known as PD-L1 (Programmed Cell Death - Ligand 1) and blocks interactions with a protein known as PD-1 which is present on T-cells. PD-1 is a protein on T-cells which plays an important role in inhibiting immune responses and regulates self-tolerance. In many cases, including pancreatic cancer, a protein known as PD-L1 exists on the tumor cells which will bind to the PD-1 protein and increase the production of T-cells with increased expression of the PD-1 protein. This way, the immune system is weakened as the increased expression of PD-1 proteins inhibits the T-cells’ ability to target tumor cells and tumor cells can easily escape and grow without being hindered by the immune system.
- As shown by the image below, Atezolizumab blocks the interactions between PD-1 and PD-L1, so that the immune response is not inhibited by the high proliferation of PD-1 expressing T-cells. This therefore weakens the tumor cells.
-
9 weeks after the surgery, the patients enrolled in the trial were given 8 consecutive priming doses of personalized vaccines, each containing 20 neoantigens per patient.
- The priming doses refer to the first of vaccine doses which contain a higher dose of the vaccine than the maintenance or booster dose. The aim of the priming dose is to “prime” or initially stimulate the immune system by introducing the neoantigens on the surface of the body’s immune cells. These doses aim to increase the activation and expansion of neoantigen-expressing immune T-cells.
-
Following this, the patients received FOLFIRINOX chemotherapy.
- FOLFIRINOX is an aggressive form of chemotherapy that is given to patients with Pancreatic Ductal Adenocarcinoma (PDAC) which has metastasized to other parts of the body.
- It is a combination of drugs:
- FOL - folinic acid.
- F – fluorouracil
- Irin - Irinotecan
- OX - Oxaliplatin
- FOLFIRINOX chemotherapy drugs mentioned above specifically target rapidly dividing cells.
- Chemotherapy is commonly used with immunotherapy as it can debulk primary tumors (sites where the tumor originated) which reduces the number of cells that the immune system has to fight against, while also reducing the immunosuppressive natures of the tumor cells.
Glossary of Terms appearing frequently throughout my project:
- Pancreatic Ductal Adenocarcinoma (PDAC): A type of pancreatic cancer caused by cancerous cells lining the pancreatic duct. This lining of cancerous cells impedes the pancreatic enzymes from flowing through the pancreas.
- Immune checkpoint inhibitors: Type of traditional immunotherapy method which works by stopping the interaction between the antigens on the tumor cells and the checkpoint proteins on immune cells.
- T-cells: These are a type of white blood cells which help to fight infection by containing a memory of previous infections and invaders to the immune system. They also send signals to the immune system to fight againt foreign elements in the body.
- Neoantigens: Types of antigens known as Tumor Specific Antigens which appear specifically on the surface of tumor cells, and are used as the primary targets for immune T-cells in mRNA vaccines.
- PD-1 (Programmed Death-1): A type of receptor on immune T-cells which regulates self-tolerance and slows down immune responses. This antigen ensures that an immune system does not attack its own "self."
- PD-L1 (Programmed Death Ligand-1): A type of antigen on the surface of tumor cells which binds/interacts with PD-1 on immune T-cells. PD-L1 increases the presence of PD-1 on T-cells and therefore decreases the immune system's ability to screen out tumors.
- RFS (Recurrence Free Survival): This is the time period that a patient is cancer-free after receiving their first round of treatement.
Data
The graph below shows the ineffectiveness of immune checkpoint inhibitors during two different trials conducted against PDAC, due to the low mutational burden of PDAC tumors, and the inability of immune checkpoint inhibitors to stimulate an immune response, unlike the mRNA vaccines. We can see that in the first row, out 27 patients who were enrolled in the clinical trial, only 27 had an immune response to the treatment. In the second row, out of 14 patients enrolled in the trial, none of them had a any response to the checkpoint inhibitor. This therefore shows that immune checkpoint inhibitors are ineffective against Pancreatic Ductal Adenocarcinoma (PDAC).
Data for the BioNtech Phase 1 trial testing autogene cevumeran (mRNA vaccine) for Pancreatic Ductal Adenocarcinoma:
- The primary endpoint of this trial testing mRNA vaccines on patients with PDAC was safety. As seen from the graph below, this endpoint was met as a small percentage (less than 10%) of the patients in the Autogene Cevumeran (name of the mRNA vaccine) faced Grade 3 Adverse Events (AEs), specifically fever and hypertension. As well, 0% of the patients had Grade 3 AEs for atezolizumab. Both of these recorded levels were well within the study threshold of 25% indicated by the blue dotted line in the graph. A grade 3 AE is considered an adverse event that requires significant medical attention, but is not life-threatening to the patient.
- The second primary endpoint of this Phase 1 trial of the Autogene cevumeran mRNA vaccine for PDAC was feasibility. Since these vaccines were manufactured in Mainz, Germany, and the patients were being treated in New York, U.S.A, it was important that the vaccine doses and the treatments were administered without delay and within the benchmarked times. From the graph below, we can see that the benchmarked times for the administration of atezolizumab as well as Autogene Cevumeran were met. The administration of these were within 3 days of the benchmarked time.
- This study also tested the immunogenicity of the neoantigen mRNA vaccines, or whether or not these vaccines were able to stimulate a response in the patients’ T cells. The study found that 8 out of the 16 patients (50%) had T-cells which were able to develop the tumor-specific neoantigens that the autogene cevumeran mRNA vaccine introduced to the patients’ immune system. These patients were classified as responders, and meet the secondary endpoint of this study which is the immunogenicity of the neoantigen mRNA vaccines is met by this.
- From the graph, it can be seen that in the responders a certain number of the neoantigens in the autogenecevumeran vaccine were immunogenic and were able to stimulate the patient's immune system (coloured yellow in the graph).
- From the plot below, we can see that the administration of the personalized neoantigen vaccines resulted in an increase of the vaccine related T-cell expansion (creation of T-cell clones) that expressed the neoantigens which were present on the tumor cells of the patient. This is in contrast to the 8 non-responders who did not have any T-cell expansion after mRNA neoantigen vaccine administration.
- Finally, as seen from the graph, vaccine non-responders (represented by the blue line) had a recurrence free survival (RFS) of 13.4 months after the surgical resection of their tumors. However, the 8 responders (represented by the red line) continue to have an RFS of 100% even after the follow-up period of 18 months. This shows that having a response to the neoantigen vaccine results correlates to delayed Pancreatic Ductal Adenocarcinoma recurrence.
Conclusion
- Until recently, traditional immunotherapy methods such as immune checkpoint inhibitors were ineffective against Pancreatic Ductal Adenocarcinoma (PDAC). The main reason for this is because pancreatic tumors have very few mutations that code for antigens, meaning that they are less likely to be caught by the immune system, and have an immunosuppressive effect. As seen from the data, past clinical trials with checkpoint inhibitors have shown that they cannot trigger an immune response in the PDAC patient's immune system.
- However, personalized neoantigen mRNA vaccines, in contrast, have shown promising results against PDAC, as these vaccines work by introducing a type of Tumor Specific Antigens known as neoantigen vaccines to the patient’s immune system, thereby allowing the immune system to recognize the Pancreatic tumor cells as “foreign.”
- The research and the data presented support my thesis that personalized mRNA neoantigen vaccines can result in better overall survival for patients with stage 1, surgically resectable Pancreatic Ductal Adenocarcinoma by allowing the immune system’s memory T-cells to recognize the tumor specific neoantigens present on the tumor cells, and attack and suppress the pancreatic tumors.
- As seen from the presented data, the mRNA vaccine autogene cevumeran tested in the Phase 1 clinical trial was able to create a strong immune response in 8 out of the 16 patients enrolled in the study, as several neoantigens in each of the vaccines for these 8 patients were immunogenic and were able to prime these patients’ immune system to recognize the tumor cells with these neoantigens as foreign and attack them. The administration of the mRNA neoantigen vaccine also was able to expand T-cells by producing T-cell clones expressing the neoantigens in the responders.
- It is also supported by the data that immune response to the neoantigen mRNA vaccine correlates with delayed PDAC recurrence (longer RFS), as the 8 responders to the neoantigen vaccine had 100% Recurrence Free Survival (RFS) for around 25 months (surpassing the follow up period of 18 months), whereas the responders had a median RFS of only 13.4 months.
- Therefore, the research and data analysis show personalized neoantigen vaccines are a safe, feasible method to treat Pancreatic Ductal Adenocarcinoma as they are able to stimulate the immune system to recognize and screen out the tumor cells, and are related to delayed recurrence of the cancer.
Future Steps:
- One future step for the administration of mRNA neoantigen vaccines for Pancreatic Ductal Adenocarcinoma is a Phase 2 clinical trial. This trial was recently approved and would involve 260 patients from 80 sites around the world. While the Phase 1 trial tested whether the mRNA vaccine would be able to trigger an immune response in patients with Pancreatic Ductal Adenocarcinoma, this new trial would compare this treatment with the current treatment for PDAC which is surgery followed by chemotherapy. The patients would be divided into a control group which would receive standard care for PDAC (surgery and chemotherapy), and a group on which mRNA cancer vaccines would be tested. This would enhance learning and understanding of the benefits that mRNA neoantigen vaccines would offer, over current treatments.
- Another future step in the field of mRNA vaccine therapy is extending this therapy to other cancers such as lung and skin cancer. A recent clinical trial in the U.K. has been launched which uses therapeutic cancer immunotherapies that are tailored to a particular type of cancer, to treat patients who have been diagnosed with lung or skin cancers. It is being tested to see its capability to shrink tumors. mRNA cancer vaccines can provide a reliable and long term cure for patients with lethal cancers. The hope is that mRNA cancer vaccines can be extended to treat all types of cancers in the near future.
Citations
- Checkpoint inhibitors. Checkpoint inhibitors | Types of immunotherapy | Cancer Research UK. (2022, February 15). https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types/checkpoint-inhibitors
- Guardian News and Media. (2024, February 4). First UK patients receive experimental messenger RNA cancer therapy. The Guardian. https://www.theguardian.com/science/2024/feb/04/first-uk-patients-experimental-messenger-mrna-cancer-therapy-treatment
- Http://journals.sagepub.com/doi/abs/10.1177/0887302x07303626 | ... (n.d.). https://www.researchgate.net/publication/328039672_httpjournalssagepubcomdoiabs1011770887302X07303626
- The pancreas and its functions. Pancreas Functions, Location & Disease | Columbia Surgery. (n.d.). https://columbiasurgery.org/pancreas/pancreas-and-its-functions
- Pancreatic Cancer Action. (2022, September 8). Distal pancreatectomy & splenectomy. https://pancreaticcanceraction.org/about-pancreatic-cancer/treatment-options-for-pancreatic-cancer/treating-pancreatic-cancer/surgery-for-operable-pancreatic-cancer/distal-pancreatectomy-splenectomy/
- Pancreatic cancer treatment. National Cancer Institute. (n.d.). https://www.cancer.gov/types/pancreatic/patient/pancreatic-treatment-pdq
- Rojas, L. A., Sethna, Z., Soares, K. C., Olcese, C., Pang, N., Patterson, E., Lihm, J., Ceglia, N., Guasp, P., Chu, A., Yu, R., Chandra, A. K., Waters, T., Ruan, J., Amisaki, M., Zebboudj, A., Odgerel, Z., Payne, G., Derhovanessian, E., … Balachandran, V. P. (2023, May 10). Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature News. https://www.nature.com/articles/s41586-023-06063-y
- Whipple procedure. Johns Hopkins Medicine. (2021, August 23). https://www.hopkinsmedicine.org/health/conditions-and-diseases/pancreatic-cancer/whipple-procedure
- YouTube. (2016, June 21). What is pd-1? ask A scientist. YouTube. https://www.youtube.com/watch?v=o2ldbt50uWc
- YouTube. (2022, June 30). Phase I trial of mrna neoantigen vaccines for pancreatic cancer. YouTube. https://www.youtube.com/watch?v=7AlMcarXdBo&t=148s
Acknowledgement
I would like to acknowledge and thank my parents for supporting me throughout my science fair project. As well, I would like to thank Henry Wise Wood High School, and my science fair coordinator Mr. Manias for providing the opportunity for me to participate in this year's Calgary Youth Science Fair.