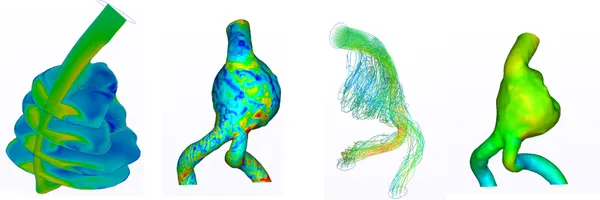
Blood Flow Study Within Abdominal Aortic Aneurysms
Grade 11
Presentation
Problem
Abdominal Aortic Aneurysms (AAAs) are the cause of over 175,000 deaths annually worldwide. The rates of mortality of these aneurysms only continues to grow, showing an overall increase of 82.1% since 1990. Furthermore, these aneurysms rarely show symptoms, most symptoms only noticeable when rupture has occurred. The overall mortality rate of AAA rupture reaches 80%-90% due to the fatal bleed that occurs afterwards. Computational Fluid Dynamics (CFD) has shown potential in terms of becoming a possible tool for rupture-risk assessment. This technology has been used in studies to investigate the hemodynamic parameters within aneurysms. By using CFD to determine blood flow patterns accurately within aneurysms, a method can be developed regarding assessing the risk of rupture.
Method
I created an educational account on Autodesk and installed Fusion 360 and Autodesk CFD. Fusion 360 is an integrated cloud software platform that includes all the tools for 3-D modeling and simulations, and Autodesk CFD is a software that facilitates computational fluid dynamics simulations which are used by engineers and analysts to predict the performance of liquids and gases [46]. These programs were used to prepare the 3D model (Fusion 360), and study the blood flow within it (Autodesk CFD).
The first step is to prepare the geometry:
1) Make a solid body from a mesh body (Figure 13).
(Figure 13)
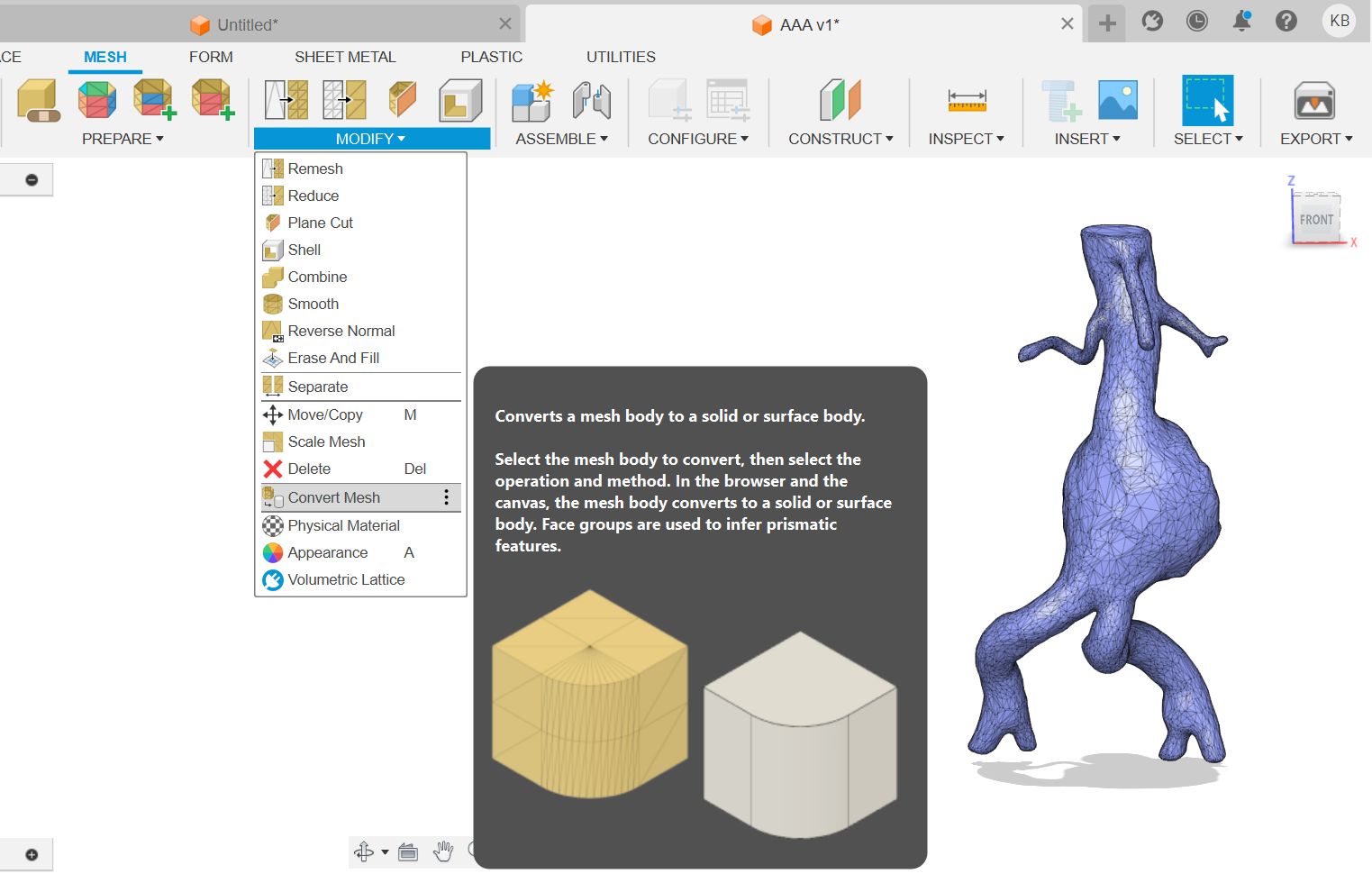
Figure 13: Transformation of mesh body into a solid body
As a result of this operation a solid body was generated out of a mesh shell which was described in the data (Figure 14).
(Figure 14)
Figure 14: Generated solid body
Since I'm going to set up the boundary conditions later, several flat surfaces need to be prepared. (Figure 15).
(Figure 15)
Figure 15: Preperation of flat surfaces.
Cutting planes were generated in order to cut the selected areas shown in (Figure 15) to create a flat surface. (Figure 16).
(Figure 16)
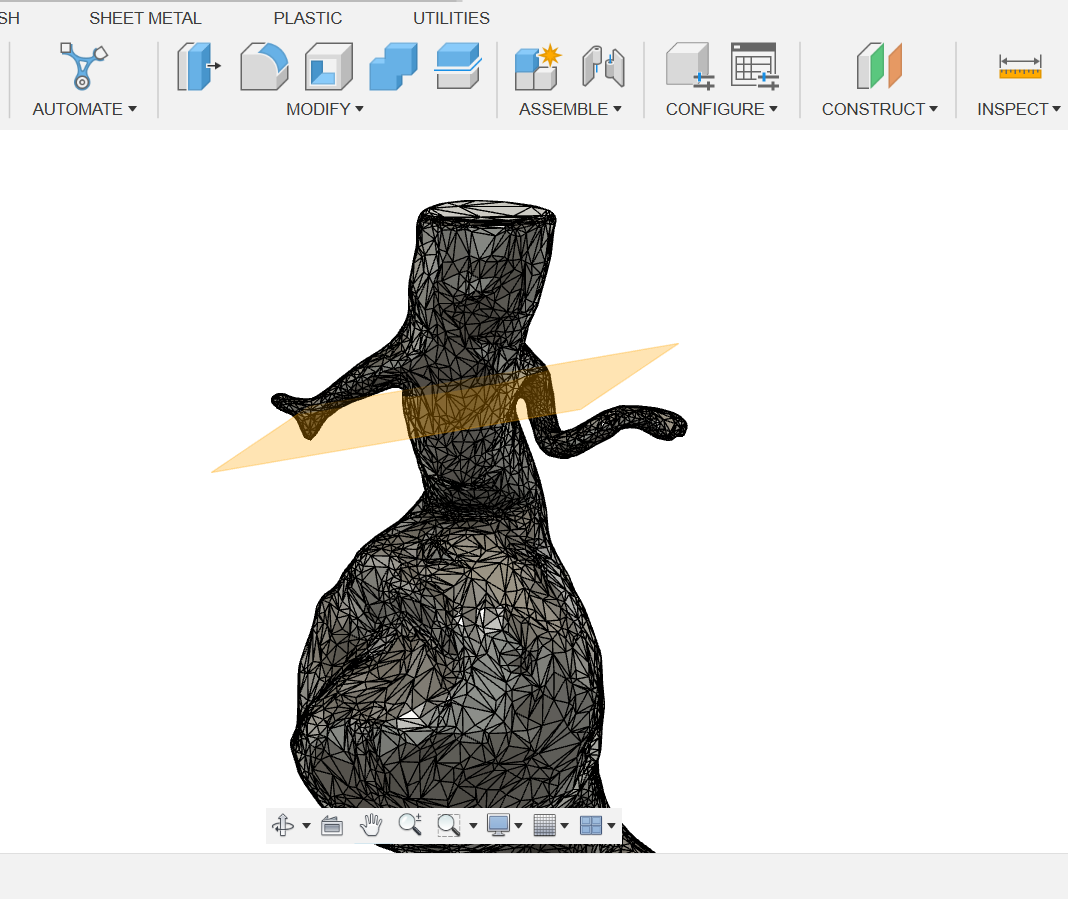
Figure 16: Created cut plane
Then I used the "split body" feature in order to make cuts with the cutting planes (Figure 17).
(Figure 17)
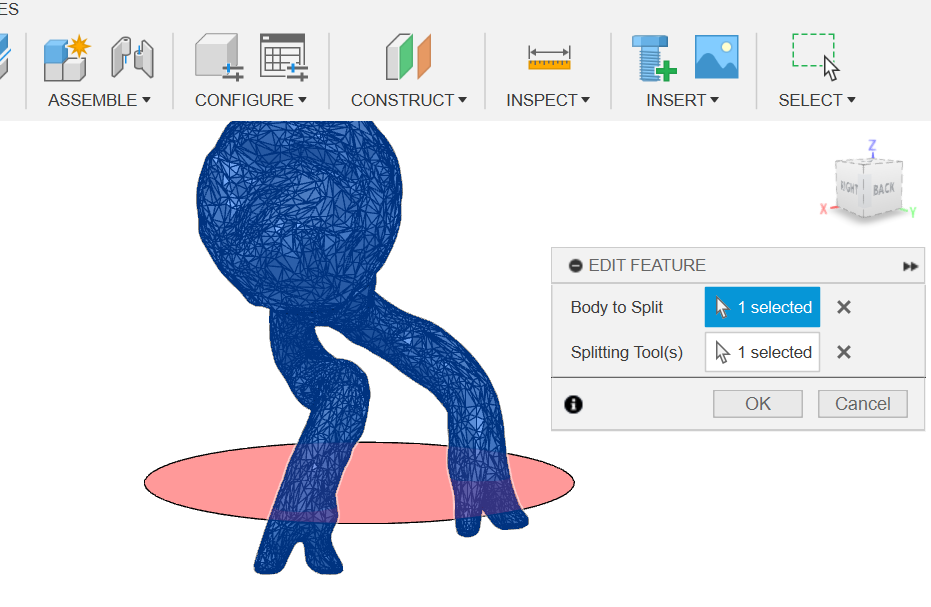
Figure 17: Splitting body with the plane
The results of the cut can be seen below in (Figure 18).
(Figure 18)
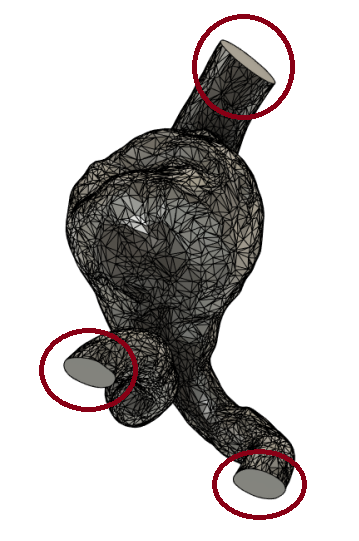
Figure 18: Results of the cut
Afterwards, the component geometry was exported from Fusion 360 as a .step file. This processed solid body geometry with flat inlet and outlets was imported to Autodesk CFD (Figure 19).
(Figure 19)

Figure 19: Imported solid body into Autodesk CFD
Firstly, the blood properties are assigned to a fluid volume out of the CFD fluids library (Figure 20). Blood is a non-Newtonian fluid, and in Autodesk CFD non-Newtonian fluid is set as a power law fluid.
(Figure 20)

Figure 20: Assigning blood properties to a fluid volume using CFD fluids library.
Next, the boundary conditions are set up. Blood flow travels down through the aorta from the top with an average velocity of 150-175 cm/sec [45], therefore 175 cm/sec was set as the velocity magnitude entering from the top of the aorta (Figure 21). On the other end, unknown boundary conditions were set (Figure 22).
Definition of unknown boundary conditions from Autodesk CFD documentation: "This is a “natural” condition meaning that boundary is open, but no other constraints are applied."
(Figure 21)

Figure 21: Set up of boundary conditions for vessel entrance.
(Figure 22)

Figure 22: Selecting unknown boundary conditions for the other end of the vessel.
Before the Autodesk CFD analysis is run, elements are created by breaking down the geometry into smaller pieces. Each element has a corner which is called a node. At the nodes, the calculations are performed. The mesh is made up of these elements and nodes. Most elements in 3-D models have a tetrahedral shapes whereas in 2-D models most elements are triangles [46].
Automatic Mesh Sizing was used to create the mesh. Automatic Mesh Sizing defines a mesh that is optimized for the model and accurately represents every detail of the geometry. For further refinement, Mesh Adaptation was used. Mesh Adaptation uses solution results to progressively improve the mesh definition. The simulation is run several times, and each time the results in the previous cycle are used to improve the mesh in the next cycle. The result is a mesh that is optimized for the particular simulation. The mesh is finer for high gradient regions, and coarser elsewhere. ( directly from Autodesk CFD documentation: https://help.autodesk.com/view/SCDSE/2024/CHS/?guid=GUID-CB2F0968-7149-4679-AC93-DD2D4070BD13 )
Two adaptive cycles were performed and the mesh was refined form approximately 1M to approximately 3.7M fluid elements.

(Figure 23)

Figure 23: Mesh structure (3.6M elements)
(Figure 24)

Figure 24: Magnified mesh structure
Lastly, solver was set to calculate steady-state solution, and the results were saved every 100 iterations. Low Re k-epsilon model was used to model turbulence, and heat transfer was not considered.
Research
Aneurysms and its main types
An aneurysm is a localized widening of an artery or vein by a minimum of 50% of its normal diameter. Aneurysms cause weakening in the affected vascular wall and can lead to rupture with potentially lethal bleeding and death. They can occur in any part of the body but are mostly seen in the arteries. The two most common types of aneurysms are the abdominal aortic aneurysm (AAA) and the cerebral aneurysm (CA). Together, these aneurysms result in over 80% of fatal aneurysm cases. The AAAs are mainly located in the infrarenal aorta and the CAs are commonly located in the circle of willis, which is a ring of vessels connecting several important arteries at the bottom part of the brain. In addition to their different locations, these aneurysms have different shapes, these shapes seen in (Figure 1). While the majority of AAAs have a fusiform shape (balloons out on all sides of the vessel), 90% of CAs have a saccular shape (balloons out only on one side) [1] [2] [3]. Less common than the AAA and CA is the thoracic aortic aneurysm, or the TAA. This type of aneurysm is located in the chest in the thoracic aorta which consists of four main segments: the ascending aorta, the aortic root, the aortic arch, and the descending aorta. The aneurysm can occur in any area in these parts [4] .This aneurysm rarely shows symptoms with approximately 95% of cases being asymptomatic. Because of this, they are referred to as "silent killers'' as they can go unnoticed to the point of an alarming consequence that can often be fatal [5].
Figure 1:
Rare Types of Aneurysms
Mycotic Aneurysm: This type of aneurysm is caused by the expansion and weakening of the arterial wall as a result of an infection. They are extremely rare (due to modern antibiotic therapies), making up only 0.7% - 3% of all arterial aneurysms. Final management requires the removal of all infected tissue by using surgical interventions [6], however non-surgical options are available if the aneurysm has been caught early or isn't at an alarming size. When this aneurysm goes untreated, it results with a fatality caused by either a big hemorrhage (loss of blood due to a damaged blood vessel) or fulminant sepsis (when the body responds poorly to an infection and death occurs within 48 hours) [7] [8]. These aneurysms are commonly caused by bacterial infections [9], however they may also be caused by a variety of fungi, mycobacteria and viruses.
Pseudoaneurysm: This aneurysm is also known as the "false aneurysm" and is caused when the arterial wall is damaged which results in a hematoma in that area with unstable blood flow. Unlike true aneurysms, pseudoaneurysms only include 1 layer of the vessel wall rather than all three like the true aneurysms. Their overall incidence is 0.6% to 4.8%. This aneurysm presents as a painful and pulsatile mass which, when enlarging, can cause pressure on the skin. Options for managing these aneurysms include ultrasound guided compression, observation, ultrasound-guided thrombin injection and surgical repair [10].
Blood Blister Aneurysms: These aneurysms make up approximately 2% of all intracranial aneurysms and are considered to be false aneurysms or dissecting aneurysms (hematomas that commonly occur in the aorta). They typically consist of a shallow focal protrusion (bump) and are located on the internal carteroid artery [11]. Their walls are weak which can be an evident characteristic of this type of aneurysm. The weakness of their walls highlight the unique appearance of these lesions as well as showcases their high rupture risk and tendency for rapid growth and progression [12]. An image of the blister aneurysm can be seen in (Figure 2).
Figure 2:
Statistics
Abdominal Aortic Aneurysm: The occurrence of rupture is approximately between 5.6 and 17.5/100,000 individuals per year. The general mortality rate of ruptured incidences of AAA is 80%-90%. [13] Throughout studies of the natural history of AAAs, it was noted that the rate of rupture and mortality could surpass 60% within 3 years of the initial diagnosis, therefore early diagnostic and surgical intervention are essential to avoid rupture [14].
Cerebral Aneurysm: The worldwide occurrence of cerebral aneurysms is approximately 3.2%. Subarachnoid hemorrhage (SAH), or bleeding in the space surrounding the brain, causes approximately 5% of all strokes, and 85% of SAHs occur as a result of aneurysmal ruptures. The occurrence rate of an SAH is 9 per 10,000 individuals per year with a mortality rate of approximately 50%. Furthermore, it was observed that 50% - 80% of all cerebral or intracranial aneurysms do not rupture throughout an individual's lifetime [15].
Thoracic Aortic Aneurysm: In general, approximately 60% of TAA cases occur in the aortic root and the ascending aorta, 40% in the descending aorta and 10% in each arch or the thoracoabdominal segment. The occurrence of TAA is 5-10 per 100,000 individuals per year [16]. Furthermore, TAAs are the 15th leading cause of death in individuals aged anywhere above 55 years and the 19th leading cause of death in individuals overall. A study has shown that nearly 40% of patients suffering from a TAA rupture reach the hospital alive [17]. Additionally, population-based studies have shown that the occurrence of acute aortic dissection (tear in the inner layer of the body's main artery) and thoracic aortic rupture is 3.5 per 100,000 individuals [18].
Symptoms
Abdominal Aortic Aneurysm: Pain in the back, abdomen or flank (area on the back or sides of the abdomen between ribs and hips) are the most common symptoms [19]. However, AAAs tend to be asymptomatic.
Cerebral Aneurysm: Sudden severe headaches along with a stiff neck, vomiting and photophobia (an extreme sensitivity to light) [15].
Thoracic Aortic Aneurysm: Symptoms of the TAA include back pain, cough, shortness of breath and tenderness or pain in the chest. If the TAA has ruptured, the symptoms could include a sharp sudden pain in the chest, arms, neck or jaw, difficulty breathing, low blood pressure and possibly even trouble swallowing [20].
Most aneurysms are asymptomatic.
Causes and Diagnosis
The majority of abdominal aortic aneurysms tend to be asymptomatic and barely detectable during physical examinations. Although the exact specific cause of aneurysms is not yet fully known, studies have shown that tobacco use (or smoking in general), hypertension (high blood pressure), obesity, older age, a family history of AAA and the male gender are potential risk factors concerning the development of an aneurysm.
One of the standard imaging tools used to detect AAAs is an ultrasound. This tool has a sensitivity and accuracy coming close to 96%-100% for the detection of infrarenal (below the kidney) AAAs [21]. An image example of an ultrasound of an aneurysm in the abdominal aorta is shown in (Figure 3). Additionally, a CT scan is used in order to determine the exact location and size along with other modern imaging technology such as PET and MRI scans which can help evaluate the risk of rupture in the aneurysm [22]. An MRI of an AAA is shown below in (Figure 4) and a computed tomography which demonstrates the normal caliber aorta (A) and the 3.7 cm dilated aorta (B) in (Figure 5) [23].
Figure 3:
Figure 4:
Figure 5:
Treatment:
Abdominal Aortic Aneurysms: For abdominal aortic aneurysms surgical intervention is required when the aneurysm has reached a diameter of 5.5 cm or greater. However, several non-surgical options for slowing down an aneurysm's growth and progression have shown potential throughout studies, one of the methods being a behavioral intervention such as quitting the habit of smoking as the habit has shown to be a possible issue regarding aneurysms, increasing its growth rate up to 0.4 mm annually [23]. Furthermore, several anti-inflammatory medications (statins, doxycycline and roxithromycin) as well as antihypertensive agents (i.e. beta-blockers (a medication that decreases high blood pressure) and diuretics) have been tested throughout studies in hopes of obtaining the ability to limit the expansion rate of a small AAA [24]. However, beta-blockers have shown no significant effect on the growth rate of abdominal aortic aneurysms, with clinical trials having been run with a dropout rate of up to 40% in the propranolol group, proving that propranolol should not be further used in the prevention of small AAAs [25]. Statins, on the other hand, have shown to slow the progression rate of AAAs. AAAs have shown to increase the risk of cardiovascular complications as a result of atherosclerotic manifestations (thickening or hardening of the arteries) in the vascular area. Throughout the duration of 5 years, the mortality rate of AAAs is 25%, mainly caused by cardiovascular events. According to a metaanalysis which studied the effect of statin treatment in patients with AAAs, statins have been able to slow the progression rate of atherosclerosis and lower the mortality rate by approximately 43% throughout a 5-year period of time. Along with the benefit statins have against cardiovascular complications, they have even shown to slow the growth rate of AAAs as they have also shown to reduce inflammation of vascular walls [26]. Overall, the non-surgical method that has shown the most potential throughout studies is the use of statins.
Surgical methods of treating AAAs: The two main types of surgical methods used to manage and treat AAAs are open repair and endovascular aneurysm repair. For open repair of an AAA, a large incision is made in the abdomen in order to get a clear view of the aortic aneurysm. Once the abdomen is open, the aneurysm wil be repaired using a graft which is a long cylinder-like tube. These grafts are made out of a variety of materials, including polytetrafluoroethylene, also known as PTFE, which is a non-textile synthetic graft, or Dacron, a textile polyester synthetic graft. The graft is stitched to the aorta, connecting one end of the aorta that is located in the aneurysm-affected area to the aorta's other end. This method is considered the standard procedure for AAA repair [27]. Endovascular aneurysm repair is considered to be a less invasive procedure for the repair of aneurysm as it is a procedure that is done from within the blood vessel without the use of open surgery. In an endovascular aneurysm repair, the aneurysm is excluded from blood circulation with the use of a device called an endograft. The endograft is sent to the location of the aneurysm with the use of a catheter that is inserted into an artery in the groin [28]. Visual representations of the open repair graft as well as the endovascular repair graft are shown in (Figure 6) and (Figure 7).
Figure 6:
Figure 7:
Fluid Properties and Classification (as specific definitions):
Compressibility refers to the change in volume (V) of a substance that is subjected to a change in pressure on it. There are two types of fluids which are considered during flow modeling: compressible fluids and incompressible fluids. Gasses, for example, are compressible fluids because they experience significant change in volume at slight changes in pressure. Incompressible fluids are the fluids which are difficult to compress. The volume of these fluids almost stays the same at large increases in pressure.
Density refers to the amount of mass per unit volume of a substance.
Specific weight is the amount of weight per unit volume of a substance.
Specific gravity is the ratio between the density of a fluid and the density of some substance (for example water) at a specific temperature. [29]
Viscosity is the resistance of a fluid (liquid or gas) to a change in shape or movement of neighboring portions relative to one another. In other words, it refers to the opposition to flow [30].
Newtonian fluids are fluids which have a constant viscosity regardless of the quantity of shear applied at a constant temperature. A linear relationship exists between the shear stress and viscosity with these fluids.
Non-newtonian fluids experience an increase or decrease, depending on the fluid, in viscosity when shear is applied. They are generally the opposite of newtonian fluids and can be described in one of four ways regarding behavior:
1) Dilatant - an increase in viscosity when shear is applied.
2) Pseudoplastic - a decrease in viscosity when shear is applied.
3) Rheopectic - an increase in viscosity when shear is applied, the change in viscosity occurring in a time-dependent manner.
4) Thixotropic - a decrease in viscosity when shear is applied, this decrease occurring in a time-dependent manner. [31]
Computational Fluid Dynamics (CFD)
CFD is a science that uses digital computer software, numerical analysis, and data structures in order to analyze and solve various problems regarding fluid flow [32]. This software analyzes various properties relating to fluid flow such as pressure, density, viscosity, physical solution, velocity, and temperature. For an accurate result, these factors are calculated concurrently [33].
Hemodynamics
In medical context, hemodynamics is often referred to as the basic measure of cardiovascular function which includes the cardiac output and arterial pressure. However, the term can also be looked upon as the physical study of flowing blood, including all the solid structures that it flows through (such as arteries) [34]. A true understanding of circulation within the human body cannot exist without a basic comprehension of basic hemodynamic principles. The basic variables used in hemodynamics are flow, volume, pressure, compliance, velocity, and resistance [35].
Blood is essentially a Non-Newtonian fluid which means that it is a fluid with a shear rate dependent viscosity. In other words, as shear rate increases, the viscosity decreases in blood. Viscosity of blood comes from the shear rate-shear stress connection. When fluids have a higher viscosity, they experience less deformation and have slower flow in comparison to fluids with a lower viscosity. Blood demonstrates a behavior known as shear-thinning meaning that a non-linear decrease is present in the viscosity while there is an increasing shear rate [36]. Therefore at normal concentrations of blood cells, which is approximately 45% volume-wise in normal human blood, the blood of mammals demonstrates slight shear thinning. This occurs mainly due to the fact that at low shear rates, red blood cells cluster together to form long strands due to the interactions with cell surface proteins and charge attraction. Unlike blood in general, the plasma held in it is Newtonian and holds a viscosity approximately twice as much as water. However, the physical behavior regarding blood changes depending on the vessel diameters, particularly when the diameters of the vessels reach that of red blood cells (which is approximately 8 μm in humans). The diameter of blood vessels can decrease from 1000 μm down to 3 μm and therefore the blood's viscosity decreases down to about a 10 μm diameter before increasing abruptly from the diameters of vessels going from 10 μm down to 3 μm. Overall, the flow of blood in smaller vessels is significantly complex [37].
Application of CFD to Aneurysms
The prediction of aneurysm ruptures tends to prove itself difficult and complex. Computational Fluid Dynamics, or CFD, is a method that has been studied for many years in hopes of figuring out how to use this technology beneficially with aneurysm evaluation. Models of this technology have been used with two main objectives, one being to identify factors regarding hemodynamics which can be used to tell apart between low and high risk aneurysms in order to develop aneurysm-evaluation systems, and the second being to comprehend the effects of different procedures and devices in hopes of improving device designs as well as select a method most appropriate for a patient with an aneurysm.
With the use of CFD, scientists have been able to identify the different variables tying to the characteristics of flow in aneurysms and therefore have been able to come up with several critical morphologic and hemodynamic parameters that have proved to have shown potential rupture risk factors. The variables discovered that tie to rupture risk include mean wall shear stress (WSS), maximum wall shear stress (MWSS), oscillatory shear index (OSI), flow, structure, pressure, the aspect ratio (AR) which is defined as the ratio between the maximum perpendicular height and the average neck diameter, and the size ratio (SR) which is the maximum aneurysm height divided by the average vessel diameter [38].
To investigate the effect on aneurysms, particularly intracranial aneurysms, CFD has proved to be a potential leading research tool for determining and investigating the effect of fluid dynamics within intracranial aneurysms and any attainable geometry. The majority of CFD studies have mainly focused on the analysis of blood flow characteristics and the effect they hold on the aneurysm wall dynamics. Studies have shown that in order to comprehend the progression of intracranial aneurysms, it is important to investigate what effects different blood viscosity models have on intra-aneurysmal hemodynamics. Additionally, most studies regarding intracranial hemodynamics have shown to depend upon the assumption that blood is a Newtonian fluid, disregarding the variations of blood viscosity. However, there are studies that do not discuss this assumption at all [39]. Furthermore, the majority of studies surrounding the role of hemodynamics in the evaluation of aneurysms come to a close agreement on the role of wall shear stress (WSS) as a significant hemodynamic parameter negatively affecting aneurysms [40][41][42].
In a clinical setting, the effectiveness of devices such as stents, flow diverters and embolic coils have also been evaluated with the use of CFD analysis. The blood flow changes were studied before device application and after in treated aneurysms [43]. Aristotelis et al. investigated the effect of coil embolization in ruptured cerebral aneurysms with the use of CFD. They studied hemodynamic parameters including blood velocity, pressure distribution on a vessel wall and blood flow patterns. An accurate 3-D computer model representation was utilized from a clinical case and an analysis was done based on the hemodynamic changes observed during the different stages of coiling processes. As a result, it was observed that the first coil influenced notable effects on blood flow patterns despite the coil only occupying 3.6% of the medium. It was also noted that gradual relief of wall pressure occurred at the aneurysm dome with a stepwise introduction of coils. However, at the aneurysm inflow zone during the intermediate coil packing, a redistribution of wall pressure was observed. This factor can be a significant tool in determining whether aneurysm regrowth and recurrence occurs after the application of the coils. In (Figure 8), the sagittal slice velocity contours can be observed within the IC aneurysm, where a is before the coil embolization, b is a simulation after the 1st deployment, c is the 7th, and d is the 15th.
(Figure 8)
Data
The 3-D abdominal aortic aneurysm model used for the numerical analysis in this project was taken from the open source: https://www.printables.com/model/81487-abdominal-aortic-aneurysm (Figure 9).
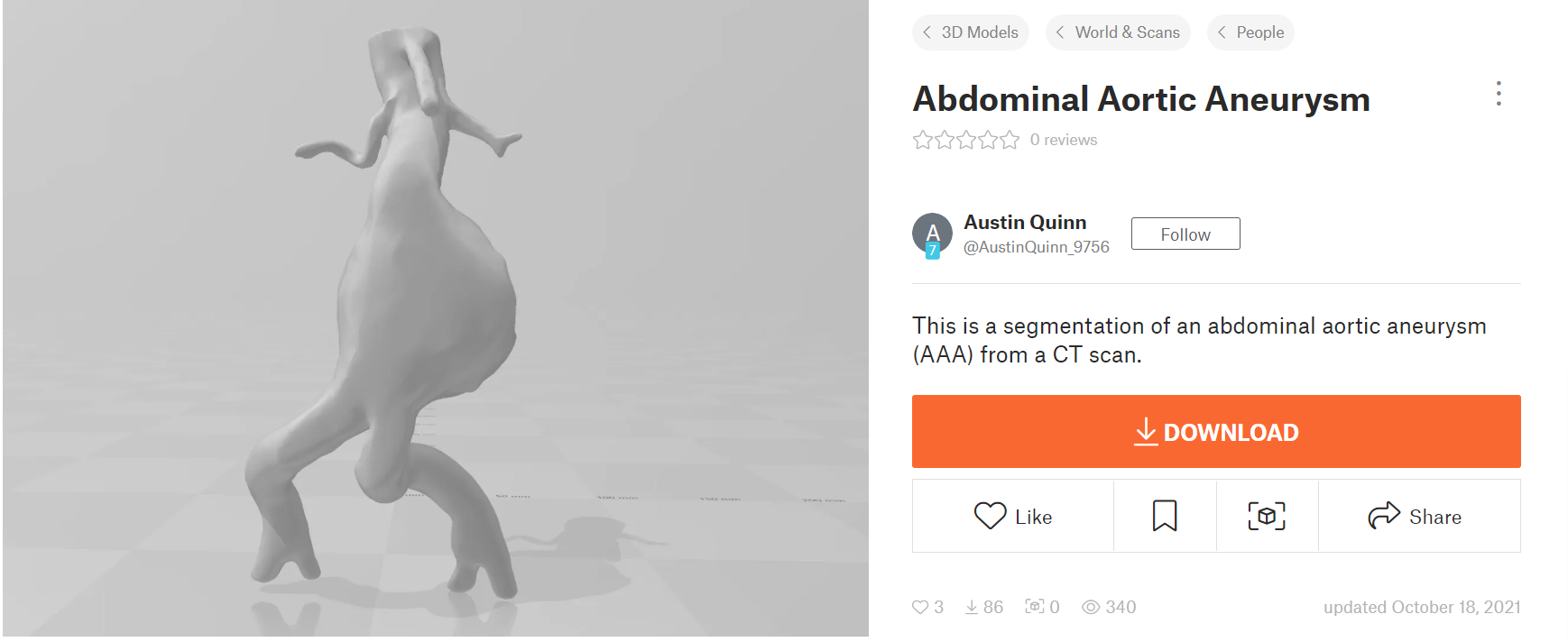
Figure 9: 3-D model of abdominal aortic aneurysm (AAA)
This aneurysm was most likely located in the infrarenal area of the abdomen, as the two vessels branching out below the aneurysm are the common iliac arteries whereas the two smaller vessels above the aneurysm lead to the kidneys (left and right renal arteries). An image representation of infrarenal abdominal aoritc aneurysms can be seen in (Figure 10).
(Figure 10)
I opened this 3D model in Autodesk Fusion 360 and inspected it. This model presented an accurate abdominal aorta diameter of approximately 2 cm. The inspection of the diameter is shown in (Figure 10).
(Figure 11)
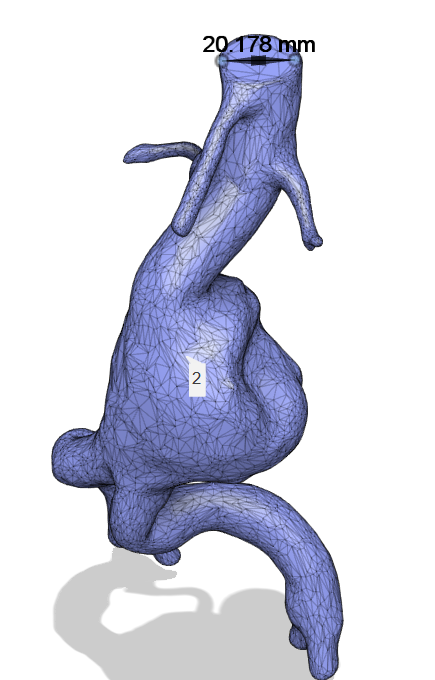
Figure 11: 3-D model open and inspected in Fusion 360.
The model is a so called mesh object, meaning that is hollow inside (Figure 11).
(Figure 12)
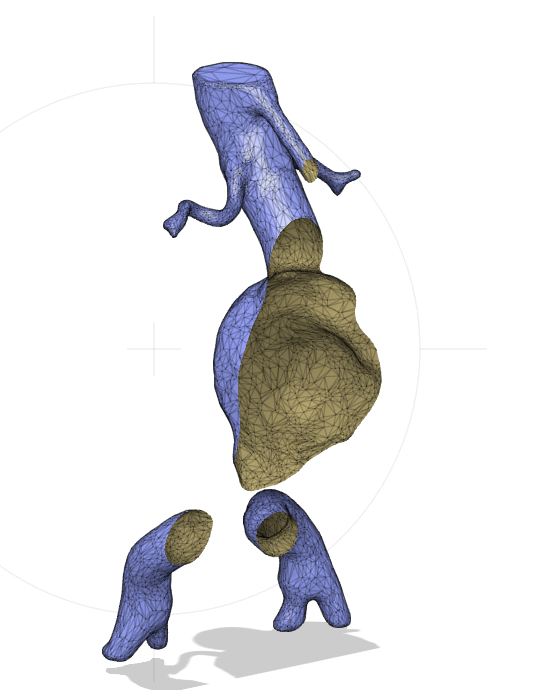
Figure 12: Section analysis
Conclusion
Results of My Calculations:
(Figure 24)

Figure 24: Traces. Velocity Magnitude.
As a result of the CFD simulation, it can be noted that the blood flow structure within the AAA (Figure 24) was interesting. As we can see, the aneurysm expansion blood flow generates a strong, low velocity vortex zone, which partially redirects the blood to one of the iliac arteries, while the rest of the flow (dominant flow) goes around this zone and significantly accelerates in the other iliac artery creating a significantly faster flow. After some research, it was found that a similar result was obtained in [47]. Furthermore, in [48], it was reported that this flow pattern occurs in 32.1% of AAA cases.
Recirculation can be better seen in different section views (Figure 25) (Figure 26).
(Figure 25)

Figure 25: Velocity magnitude vertical section.
(Figure 26)

Figure 26: Velocity magnitude for horizontal sections.
Such a flow pattern was correlated with the intraluminal thrombus (ILT) deposition in [49]. An intraluminal thrombus (ILT) is a blood clot, which consists of blood cells, platelets, blood proteins, and cellular debris. It forms between circulating blood and the aorta wall, and is present in 74% of AAAs [50-52]. Low velocity blood recirculation increases the possibility of an ILT forming. As it was stated in [49] the ILT layer can potentially cause hypoxia and prevent oxygen diffusion. Such a scenario could result in the deterioration of the posterior aortic wall, which is where aortic rupture typically occurs [53].
Wall shear stress (WSS) is a measure of the force exerted parallel to the surface of a blood vessel or artery. It is caused by the viscosity of the fluid and the velocity gradient near the wall and represents the frictional force per unit area between the fluid and the surface. WSS distribution inside AAA is shown in (Figure 27)
(Figure 27)

Figure 27: Wall Shear Stress (WSS) pattern
WSS in (Figure 27) clearly correlates with blood flow dynamics. The first region of elevated WSS is associated with the blood flow impinging the artery’s wall. Furthermore, we can see an elevated WSS along the dominant flow and low WSS zone in the region of slow recirculation.
In general, WSS is an important hemodynamic factor. It was shown in [49] that high WSS from the impingement of vortexes is associated with an absence of ILT deposition, and that impingement could have some minor influence on the AAA growth over a significant time span.
On the other hand, low WSS might be a more accurate parameter than a maximum diameter in terms of predicting the rupture of AAAs. [54][55]
Additionally, I plotted static pressure. It is interesting to note that we have two slightly elevated pressure regions, the first one being in the zone of the impinging region and the second being in the zone where the flow is hitting the wall of the aneurysm at the bottom. An elevated pressure was also noticed between the two regions. Most likely, this is related to the fact that the blood flow is pushing against the wall. In the left common iliac artery, it can be observed that there is a decrease in the static pressure because of the flow acceleration (Bernoulli's principle). This push might explain the statement above regarding the effect of impingement on AAA growth because it is stretching it by pushing against the wall.

Even though my results correlate well with literature qualitatively and quantitatively I would like to report several issues and errors which may have affected the accuracy of my numeric calculations:
Issues
Autodesk CFD is not a mainstream software and it doesn’t run well on Windows 10. I had one successful outcome out of approximately 5-6 runs. My computer often froze during the runs and Autodesk CFD was shutting down, outputting different errors. I had to reboot the computer since it was still running the calculation server, and while the server was running, it was not possible to reopen Autodesk CFD. Furthermore, it was not using Ryzen processor (32 core) efficiently, not fully loading it and RAM. Each successful run was around 24 hours. Additionally, Autodesk CFD can not export data properly for postprocessing, and all postprocessing had to be done inside or in the Autodesk CFD Viewer, which is extremely primitive. There are several exporting options, but none of them work properly.
Sources of Errors
1) Mesh Independence
In general, the finer the mesh, the more accurately we can capture flow pattern and different physical effects. A mesh independence (or grid independence) study is something we must perform to determine the dependence of the results on the mesh density and structure. Mesh adaptation, which was mentioned in the Method section, serves the role of independence study. But, unfortunately, I couldn’t run further mesh refinement after the 3.6M elements since Autodesk CFD was freezing. At the conclusion of each step, Autodesk CFD reports the mesh independence status in the Output bar (see in Method section), and the value for the pressure is not that high (83.75%). Ideally, manual refinement is needed, but I do not have enough experience and knowledge to run such advanced meshing operations.
2) Convergence
CFD simulations solve complex mathematical equations iteratively. It starts with an initial guess for the solution and then it gradually refines it through multiple iterations. Convergence is achieved when the solution is not changing anymore. I used some default criteria for convergence which looks quite loose since the choice was made in favor of the speed of calculations:

Conclusion:
1. A thorough, comprehensive review was done regarding abdominal aortic aneurysms as well as how computational fluid dynamics can and has been applied to the aneurysm rupture-risk study.
2. A 3D model of an abdominal aortic aneurysm was found and optimized for CFD analysis in Fusion 360.
3. Using Autodesk CFD, the CFD case was set and the results were obtained and processed in Autodesk CFD software.
4. Flow patterns were connected to abdominal aortic aneurysm rupture and formation of the intraluminal thrombus.
5. The results obtained demonstrated a good agreement with past studies.
Future Work:
1. Research more robust software to run CFD cases with the ability to export data properly for further postprocessing.
2. Investigate the abilities of NVIDIA Modulus to run blood flow calculations. Modulus (previously referred to as SimNet) is a framework for developing physics machine learning neural network models. https://developer.nvidia.com/modulus
An attempt has already been made with this software to calculate the full velocity field within a blood vessel aneurysm. With the use of Modulus, medical researchers can get a far better estimation of the velocities, pressures, and wall shear stresses in the blood vessel, which can help lead to better, more personalized treatment
https://docs.nvidia.com/deeplearning/modulus/modulus-v2209/user_guide/intermediate/adding_stl_files.html. In the future, I would like to use this software to further analyze blood flow within aneurysms.
Citations
[1] A.N. Sidawy, B.A. Perler, Rutherford’s vascular surgery and endovascular therapy, Elsevier Health Sciences, 2018
[2] J.C. Lasheras, The biomechanics of arterial aneurysms, Annu Rev Fluid Mech 39 (1) (2007) 293 319, https://doi.org/10.1146/annurev.fluid.39.050905.110128.
[3] John Friesen, Jonas Bergner, Mohammad Ibrahim Aftab Khan, Stefan Triess, Andreas Zoll, Peter F. Pelz, Farzin Adili, Comparison of existing aneurysm models and their path forward, Computer Methods and Programs in Biomedicine Update, Volume 1, 2021, 100019, ISSN 2666-9900, https://doi.org/10.1016/j.cmpbup.2021.100019
[4] Salameh MJ, Black JH, Ratchford EV. Thoracic aortic aneurysm. Vascular Medicine. 2018;23(6):573-578. doi:10.1177/1358863X18807760
[5] Saeyeldin AA, Velasquez CA, Mahmood SUB, Brownstein AJ, Zafar MA, Ziganshin BA, Elefteriades JA. Thoracic aortic aneurysm: unlocking the "silent killer" secrets. Gen Thorac Cardiovasc Surg. 2019 Jan;67(1):1-11. DOI: 10.1007/s11748-017-0874-x
[6] Majeed H, Ahmad F. Mycotic Aneurysm. 2023 Jul 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 32809571.
[7] Oderich GS, Panneton JM, Bower TC, Cherry KJJr, Rowland CM, Noel AA, et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results, J Vasc Surg, 2001, vol. 34 (pg. 900-8)
[8] M. Fisk, L.F. Peck, K. Miyagi, M.J. Steward, S.F. Lee, M.B. Macrae, S. Morris-Jones, A.I. Zumla, D.J.B. Marks, Mycotic aneurysms: a case report, clinical review and novel imaging strategy, QJM: An International Journal of Medicine, Volume 105, Issue 2, February 2012, Pages 181–188, https://doi.org/10.1093/qjmed/hcq240
[9] Ribé Bernal, L., Requejo, L., Ribes, A., & Miralles, M. (2020). Mycotic Aortic Aneurysms. IntechOpen. doi: 10.5772/intechopen.86328
[10] Rivera PA, Dattilo JB. Pseudoaneurysm. [Updated 2022 Mar 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542244/
[11] Nakagawa F, Kobayashi S, Takemae T, et al. Aneurysms protruding from the dorsal wall of the internal carotid artery. Journal of Neurosurgery. 1986;65:303-308
[12] I. Papasilekas, T., M. Themistoklis, K., I. Korfias, S., & E. Sakas, D. (2020). Blister Aneurysms. IntechOpen. doi: 10.5772/intechopen.89284
(Figure 2) https://thejns.org/focus/view/journals/neurosurg-focus/42/6/article-pE12.xml
[13] Maltha, M., Visser, A., Sandjer, T., Jahrome, A. K., Vink, T. W. F., & ter Avest, E. (2016). When to activate a multidisciplinary team for an acute abdominal aortic aneurysm? The American Journal of Emergency Medicine, 34(8), 1519–1523. DOI: 10.1016/j.ajem.2016.05.006
[14] Droc, I., Droc, G., Buzila, C., & Calinescu, F. B. (2018). Abdominal Aortic Aneurysms (AAA). New Approaches to Aortic Diseases from Valve to Abdominal Bifurcation, 393–402. doi:10.1016/b978-0-12-809979-7.00034-1
[15] Zhou, S., Dion, P. A., & Rouleau, G. A. (2018). Genetics of Intracranial Aneurysms. Stroke, 49(3), 780–787. doi: 10.1161/strokeaha.117.018152
[16] Mehrabi Nasab, E., Athari, S.S. The prevalence of thoracic aorta aneurysm as an important cardiovascular disease in the general population. J Cardiothorac Surg 17, 51 (2022). https://doi.org/10.1186/s13019-022-01767-0
[17] Kuzmik, G. A., Sang, A. X., & Elefteriades, J. A. (2012). Natural history of thoracic aortic aneurysms. Journal of Vascular Surgery, 56(2), 565–571. doi:10.1016/j.jvs.2012.04.053
[19] Kent, K. C. (2014). Abdominal Aortic Aneurysms. New England Journal of Medicine, 371(22), 2101–2108. doi:10.1056/nejmcp1401430
[20] https://www.mayoclinic.org/diseases-conditions/thoracic-aortic-aneurysm/symptoms-causes/syc-20350188
[21] Gilbert R. Upchurch, Jr., M.D., and Timothy A. Schaub, M.D. (2006). Abdominal Aortic Aneurysm. American Family Physician, 73(7) 1124-1297
[22] Sakalihasan, N., Michel, JB., Katsargyris, A. et al. Abdominal aortic aneurysms. Nat Rev Dis Primers 4, 34 (2018). https://doi.org/10.1038/s41572-018-0030-7
(Figure 3) Image | Radiopaedia.org
(Figure 4) Keisler B, Carter C. Abdominal aortic aneurysm. Am Fam Physician. 2015 Apr 15;91(8):538-43. PMID: 25884861.
[23] Keisler B, Carter C. Abdominal aortic aneurysm. Am Fam Physician. 2015 Apr 15;91(8):538-43. PMID: 25884861.
[24] Guessous, I., Periard, D., Lorenzetti, D., Cornuz, J., & Ghali, W. A. (2008). The Efficacy of Pharmacotherapy for Decreasing the Expansion Rate of Abdominal Aortic Aneurysms: A Systematic Review and Meta-Analysis. PLoS ONE, 3(3), e1895. doi:10.1371/journal.pone.0001895
[25] Weiss, N., Rodionov, R. N., & Mahlmann, A. (2014). Medical management of abdominal aortic aneurysms. Vasa, 43(6), 415-421.
[26] Azar Hosseini, Toktam Sahranavard, Željko Reiner, Tannaz Jamialahmadi, Yusra Al Dhaheri, Ali H. Eid, Amirhossein Sahebkar,Effect of statins on abdominal aortic aneurysm,European Journal of Pharmaceutical Sciences,Volume 178,2022,106284,ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2022.106284.
[27] Stanford Health Care. (n.d.). Abdominal Aortic Aneurysm (AAA) Open Repair. Retrieved from https://stanfordhealthcare.org/medical-conditions/blood-heart-circulation/abdominal-aortic-aneurysm/treatments/abdominal-aortic-aneurysm-open-repair.html
[28] Medical Advisory Secretariat. Endovascular repair of abdominal aortic aneurysm: an evidence-based analysis. Ont Health Technol Assess Ser. 2002;2(1):1-46. Epub 2002 Mar 1. PMID: 23074438; PMCID: PMC3387737.
[29] Mott, R. L. (2005). Applied Fluid Mechanics (6th ed.)
[30] Britannica, T. Editors of Encyclopaedia (2024, February 1). viscosity. Encyclopedia Britannica. https://www.britannica.com/science/viscosity
[31] Crane Engineering. (n.d.). What are Newtonian and non-Newtonian fluids? Crane Engineering Blog. https://blog.craneengineering.net/what-are-newtonian-and-non-newtonian-fluids
[32] Grétar Tryggvason, Chapter 6 - Computational Fluid Dynamics, Editor(s): Pijush K. Kundu, Ira M. Cohen, David R. Dowling, Fluid Mechanics (Sixth Edition), Academic Press, 2016, Pages 227-291, ISBN 9780124059351, https://doi.org/10.1016/B978-0-12-405935-1.00006-X
[33] What is CFD: What is Computational Fluid Dynamics?. SimScale. (2023, December 7). https://www.simscale.com/docs/simwiki/cfd-computational-fluid-dynamics/what-is-cfd-computational-fluid-dynamics/
[34] Secomb, T. W. (2016). Hemodynamics. Comprehensive physiology, 6(2), 975.
[35] Belloni, F. L. (1999). Teaching the principles of hemodynamics. Advances in Physiology education, 277(6), S187.
[36] Alexy, T., Detterich, J., Connes, P., Toth, K., Nader, E., Kenyeres, P., ... & Simmonds, M. J. (2022). Physical properties of blood and their relationship to clinical conditions. Frontiers in Physiology, 13, 906768.
[37] David E. Alexander, Chapter 4 - Biological Materials Blur Boundaries, Editor(s): David E. Alexander, Nature's Machines, Academic Press, 2017, Pages 99-120, https://doi.org/10.1016/B978-0-12-804404-9.00004-9
[38] Chung, Bong Jae and Cebral, Juan Raul, "CFD for Evaluation and Treatment Planning of Aneurysms: Review of Proposed Clinical Uses and Their Challenges" (2014). Department of Applied Mathematics and Statistics Faculty Scholarship and Creative Works. 26. https://digitalcommons.montclair.edu/cgi/viewcontent.cgi?article=1025&context=appliedmath-stats-facpubs
[39] Mahrous, S. A., Sidik, N. A. C., & Saqr, K. M. (2020). Newtonian and non-Newtonian CFD models of intracranial aneurysm: a review. CFD Letters, 12(1), 62-86.
[40] Wang, F., Xu, B., Sun, Z., Wu, C., & Zhang, X. (2013). Wall shear stress in intracranial aneurysms and adjacent arteries. Neural regeneration research, 8(11), 1007–1015. https://doi.org/10.3969/j.issn.1673-5374.2013.11.006
[41] Staarmann, B., Smith, M., & Prestigiacomo, C. J. (2019). Shear stress and aneurysms: a review. Neurosurgical focus, 47(1), E2.
[42] Zhou, G., Zhu, Y., Yin, Y., Su, M., & Li, M. (2017). Association of wall shear stress with intracranial aneurysm rupture: systematic review and meta-analysis. Scientific reports, 7(1), 5331.
[43] Murayama, Y., Fujimura, S., Suzuki, T., & Takao, H. (2019). Computational fluid dynamics as a risk assessment tool for aneurysm rupture. Neurosurgical Focus FOC, 47(1), E12. https://doi.org/10.3171/2019.4.FOCUS19189
[44] Mitsos, A.P., Kakalis, N.M.P., Ventikos, Y.P. et al. Haemodynamic simulation of aneurysm coiling in an anatomically accurate computational fluid dynamics model: technical note. Neuroradiology 50, 341–347 (2008). https://doi.org/10.1007/s00234-007-0334-x
(Figure 8) Mitsos, A.P., Kakalis, N.M.P., Ventikos, Y.P. et al. Haemodynamic simulation of aneurysm coiling in an anatomically accurate computational fluid dynamics model: technical note. Neuroradiology 50, 341–347 (2008). https://doi.org/10.1007/s00234-007-0334-x
(Figure 10) https://www.sjchs.org/living-smart-blog/blog-details/blog/2020/02/18/what-is-an-abdominal-aortic-aneurysm
[45] Liu, D., Fan, Z., Li, Y. et al. Quantitative Study of Abdominal Blood Flow Patterns in Patients with Aortic Dissection by 4-Dimensional Flow MRI. Sci Rep 8, 9111 (2018). https://doi.org/10.1038/s41598-018-27249-9
[46] https://help.autodesk.com/view/SCDSE/2023/ENU/?guid=GUID-BE8C3B02-3613-4237-AD18-033FE3002C34
[47] Zhou, Zhijun & Teng, Biyun & Zhao, Yu & Wang, Zhe. (2021). Comparison of small symptomatic and asymptomatic abdominal aortic aneurysms based on computational fluid dynamics analysis. Medicine. 100. e27306. 10.1097/md.0000000000027306.
[48] Yue Qiu, Jiarong Wang, Jichun Zhao, Tiehao Wang, Tinghui Zheng, Ding Yuan, Association Between Blood Flow Pattern and Rupture Risk of Abdominal Aortic Aneurysm Based on Computational Fluid Dynamics, European Journal of Vascular and Endovascular Surgery, Volume 64, Issues 2–3, 2022, Pages 155-164, ISSN 1078-5884, https://doi.org/10.1016/j.ejvs.2022.05.027.
[49] Boyd AJ, Kuhn DC, Lozowy RJ, Kulbisky GP. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg 2016;63:1613–9.
[50] Almijalli, M. Does the Intraluminal Thrombus Provoke the Rupture of the Abdominal Aortic Aneurysm Wall? Appl. Sci. 2021, 11, 9941. https://doi.org/10.3390/app11219941
[51] Vorp, D.A.; Lee, P.C.; Wang, D.H.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299.
[52] Harter, L.P.; Gross, B.H.; Callen, P.W.; Barth, R.A. Ultrasonic evaluation of abdominal aortic thrombus. J. Ultrasound Med. 1982, 1, 315–318.
[53] Hans, S. S., O. Jareunpoon, M. Balasubramaniam, and G. B. Zelenock. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 41:584–588, 2005.
[54] Teng B, Zhou Z, Zhao Y, Wang Z. Combined Curvature and Wall Shear Stress Analysis of Abdominal Aortic Aneurysm: An Analysis of Rupture Risk Factors. Cardiovasc Intervent Radiol. 2022 Jun;45(6):752-760. doi: 10.1007/s00270-022-03140-z. Epub 2022 Apr 12. PMID: 35415808; PMCID: PMC9117347.
[55] Qiu Y, Yuan D, Wen J, Fan Y, Zheng T. Numerical identification of the rupture locations in patient-specific abdominal aortic aneurysmsusing hemodynamic parameters. Comput Methods Biomech Biomed Eng. 2018;21(1):1–12. doi: 10.1080/10255842.2017.1410796.
Acknowledgement
I would like to acknowledge Anne Duguay, the science fair coordinator at our school, for her assistance with the CYSF registration, as well as her support on my project.