Are the effects of different antibiotics identical on gram-positive and gram-negative bacteria?
Grade 9
Presentation
Hypothesis
If the type of bacteria (Gram-Positive or Gram-Negative) that is being tested changes, the effects of the different antibiotics will differ because various bacilli vary in defense and structure. Gram-negative bacteria has a thin, double cell membrane wall that is strong and does not absorb substances easily; Gram-Positive bacteria has a single thick membrane that is susceptible to absorbing all substances, making it easier to kill. Various antibiotics were also engineered to target and affect different bacilli in varied ways. To conclude, the effects of different antibiotics on varied bacteria will not be identical due to differences in cell wall structure, membrane permeability, and antibiotic susceptibility.
Research
Bacteria is a single-celled organism that divides into huger populations through Binary Fission. This is when it splits into 2 per a certain amount of time. There is good and bad bacteria. The good bacteria is just called bacteria, and it helps the body in different ways, such as digestion, nutrient absorption, and more. The bad bacteria is called bacilli, meaning it causes disease (Google Search, 2024). Under the umbrella of bacteria, there are 2 main types of bacteria: Gram-Positive and Gram-Negative. The main differences are their cell membranes. Gram-Positive has a much thicker cell membrane that easily absorbs substances, making it easier to kill; Gram-Negative has a thinner, but stronger and double cell membrane that does not easily absorb substances, making it harder to kill (WebMD, 2023).
Gram-Positive bacteria is a very common disease-causing bacterium. Most common diseases today are caused by it, such as strep throat. There are diverse types of this bacteria, which also include "good" bacteria in the body. Very easy to kill because its membrane easily absorbs substances (Steward, 2019). Gram-Negative bacteria is the rarer disease-causing bacteria. It is much harder to kill with antibiotics, and when disturbed, release toxins that worsen symptoms. They cause hard to treat diseases like pneumonia. There locations vary by little. In the common household, Gram-Positive can be found in dry areas, such as skin and kitchen counters (Nunez, 2019). Gram-Negative is found in damp areas, like the kitchen sink or wet, dirty dishes. These locations are not 100% guaranteed of that bacteria because they can be moved through human interference (US Micro Solutions, 2024).
Antibiotics are medications designed to kill bacteria (WebMD, 2024). Not just bacilli, but they kill all bacteria, including the "good bacteria" in your body. That is why you may experience stool changes after taking antibiotics. They kill bacteria by destroying the cell wall or interfering with the bacteria's ability to reproduce. There are 2 types of antibiotics: Broad-spectrum, kill more than one types of bacteria; Narrow-spectrum, kill only one type of bacteria (WebMD, 2024). In our current healthcare system, to kill bacteria, there are 4 main/common types of antibiotics that are targeted at either targeted at Gram-Positive or Negative:
1) Penicillin: targeted at Gram-Positive only. Narrow-Spectrum.
2) Ampicillin: targeted at both Gram-Positive and Negative. Broad spec.
3) Neomycin: targeted at Gram-Negative only. Narrow-Spectrum.
4) Erythromycin: target at Gram-Positive and some negative. Broad spec.
Although antibiotics are helping save many lives, there is a problem, called "Antibiotic Resistance." (HealthyChildren.org, 2024). Antibiotic resistance is when bacteria starts to get used to antibiotics and get resistant to them, making "antibiotic resistant bacteria." It occurs when a person takes a bigger than recommended dosage of antibiotics.
Variables
Manipulated variable:
-
The manipulated variable is the bacteria that is being tested on: Gram-Positive and Gram-Negative bacilli.
- It is the thing that is changing. The antibiotics are staying the same, but I am changing what they are tested on to see if their effects are identical.
Responding variable:
-
The responding variable is the diameter of the inhibition growth around the antibiotics in cm, which will be measured using a ruler.
- It is the thing that is responding to the change of the manipulated variable (bacteria). The antibiotic effects will change and be collected in cm.
Controlled variable:
|
Controlled variables Variables that need to be maintained throughout the experiment for best results. |
Condition: What should be controlled throughout the project? |
Why: Why does that condition need to be maintained? |
How: How are you going to control that condition? |
|
Temperature |
Being exposed to different types of temperature can affect bacterial growth. Some excel while some die, affecting results. |
Control heaters in the house so same temperature is maintained throughout home. |
|
|
Antibiotics |
Different antibiotics can vary hugely, and their effects through the 3 trials may fluctuate, interfering with accurate results. |
Only use the 4 types of antibiotics and do not change the type to a new one. Keep consistent with only the 4 antibiotics throughout the experiment. |
|
|
Concentration of antibiotics |
If one bacterium had a higher concentration of antibiotic than another, it may have more inhibition. If concentration of the antibiotic differs in the specimens, the results will vary and not be accurate. |
Only using antibiotic discs, which comes with a set amount of concentration. In the discs, the same amount of antibiotics are maintained. I will only use one type of disc for each petri-dish testing. |
Procedure
Methods (my experimental design):
Antibiotic testing:
- Prepare the agar according to the instructions on the packaging. Pour until the bottom of 2 dishes are covered and quickly put the lids back on. Let them harden.
-
Sterilize the inoculating needle. Then take some of one gram-positive bacteria and lightly zigzag on both petri-dishes.
-
Put one type of each disc in the bacteria at a good distance away from each other in one petri-dish. Label this dish A1+. Label the dish without the antibiotics C1+. This is going to be your trial one controlled dish to compare with the trial one gram-positive trial one.
-
Repeat for the trial one gram-negative bacteria, replacing the plus signs with a minus sign.
-
Take a picture every 24 hours. Each day, measure the diameter of the circle the antibiotic disc has inhibited. Take down any qualitative observations. Compare the consecutive controlled dishes and note down similarities/differences. This is crucial for differences and explaining the effects of the antibiotics.
-
Repeat steps 1-5 of antibiotic testing for Trials 2-3 for both gram-negative and positive bacteria. Remember to replace the 1 with 2 and 3 for their consecutive trial #, and the positive/negative signs for gram-positive and negative bacteria. Don’t forget the controlled slides of each trial.
-
After doing each trial and noting down observations for each, think and write final analysis concluding your results, and then do your conclusion with the answer to the problem, referring to your data doing so.
-
To dispose, put some bleach in it (powder or liquid), cover and seal, put it in a plastic bag, and throw away.
Procedure (helped from online/researched method of doing); all steps to project doing:
Bacterial-growth process:
-
Make sure you have all the materials necessary for the staining process; 2 petri-dishes, liquid agar, sharpie or marker & tape, cotton swabs, and a camera. To prepare the agar, follow the instructions on the back of its packaging.
-
With the prepared agar, pour it into the 2 petri-dishes. Fill it to just until it covers the bottom of the dish. Quickly put the lid back on to avoid contamination from the air. Let it cool for about an hour, when it turns solid.
-
Recall that gram-positive bacteria normally like dry places and gram-negative likes damp places. With one cotton swab, rub it on your skin or non-clean counter and rub it in a zig zag pattern across one petri-dish. Turn the dish and do it again for maximum distribution, and quickly put the lid back on. Label where you got the bacteria from on the dish with sharpie. Take a picture for future comparison.
-
Perform step 3, doing the cotton swab collection from a damp place such as a kitchen sink.
-
Wait for a few days while the bacteria grow. Take a picture of each petri-dish every 24 hours for future reference/comparison. You should start using the bacteria after 3-5 days of growth or until it looks clearly matured.
Gram-staining process:
-
Sterilize the inoculating needle by passing it through a candle flame a few times. Let it cool for about 5-7 seconds.
-
Put 1 drop of distilled water on microscope slide. With the inoculating needle, transfer some of the bacteria from one dish onto the microscope slide. With another microscope slide’s edge, scrape or press on the specimen smear, making it very thin. Let the specimen air dry, and quickly pass it through a candle flame to heat fix it. Don’t stop, and don’t let it get too hot. Do it until the water evaporates.
-
Put the microscope slide on a few layers of paper towels. Cover the specimen with 1—2 drops of the Crystal Violet stain for 60 seconds, and gently wash off with slow running water. Cover the specimen with a few drops of Gram’s iodine for 60 seconds and then gently wash off like for the crystal violet stain.
-
Tilt the slide a bit and drop the ethyl alcohol drop by drop in a way it covers the whole specimen. Drop until the running alcohol is no longer colored. Then, gently wash the specimen with running water.
-
Then, cover with safranin stain for 60 seconds, and then gently wash with water. Put a paper towel gently on the bacteria so it absorbs the water, and then put a cover slip on it.
-
Now, look under the microscope. Gram-positive cells would be purple; the retained the color. Gram-negative cells would be pink or red; the purple stain was washed away by the alcohol and replaced by the safranin stain. Label the petri-dish it was taken from.
-
Repeat the whole gram-staining process for the other petri-dish of bacteria.
Antibiotic testing
-
Prepare the agar according to the instructions on the packaging. Pour until the bottom of 2 dishes are covered and quickly put the lids back on. Let them harden.
-
Sterilize the inoculating needle. Then take some of one gram-positive bacteria and lightly zigzag on both petri-dishes.
-
Put one type of each disc in the bacteria at a good distance away from each other in one petri-dish. Label this dish A1+. Label the dish without the antibiotics C1+. This is going to be your trial one controlled dish to compare with the trial one gram-positive trial one.
-
Repeat for the trial one gram-negative bacteria, replacing the plus signs with a minus sign.
-
Take a picture every 24 hours. Each day, measure the diameter of the circle the antibiotic disc has inhibited. Take down any qualitative observations. Compare the consecutive controlled dishes and note down similarities/differences. This is crucial for differences and explaining the effects of the antibiotics.
-
Repeat steps 1-5 of antibiotic testing for Trials 2-3 for both gram-negative and positive bacteria. Remember to replace the 1 with 2 and 3 for their consecutive trial #, and the positive/negative signs for gram-positive and negative bacteria. Don’t forget the controlled slides of each trial.
-
After doing each trial and noting down observations for each, think and write final analysis concluding your results, and then do your conclusion with the answer to the problem, referring to your data doing so.
-
To dispose, put some bleach in it (powder or liquid), cover and seal, put it in a plastic bag, and throw away.
Observations
Observations:
|
Observations of the substances before experiment |
Gram-positive bacteria |
Again, more green bacteria were seen. Today, some of the green was starting to grow taller. It was thicker. It is looking like the bacteria is going to be green. |
|
Gram-negative bacteria |
The bacteria look the same as yesterday. Very small, grey/white, and dense/populating. Today, in the light, whole culture looked faint green. |
|
|
Crystal Violet Stain |
Runny, purple liquid with a few small bubbles in. Very low viscosity. |
|
|
Gram’s Iodine |
Slightly low viscosity, light red/magenta liquid. Occasional bubbles seen. |
|
|
Safranin Counter Stain |
Very viscous, thick dark-red liquid with a lot of bubbles in it. |
|
|
Ethyl Alcohol |
Clear, colorless low viscosity liquid. Runny! |
|
|
Solid agar |
Foggy, translucent solid with a little bit of liquid running on top. Light yellow with a few chunks of brown here and there. Odor could not be detected. |
Bacteria growth (Gram-Positive bacilli) observations:
|
Bacteria: |
Days #: |
Qualitative Observations: |
|
Kitchen Counter and Skin (Re-growth) |
Day 1 |
Very small air bubbles, possible signs of bacterial growth seen. Small, white-grey spots seen. |
|
Day 2 |
Very similar to yesterday. No signs of bacterial growth except for small, white-grey spots and a few additional bumps that looked clear. |
|
|
Day 3 |
Small-big, white-grey bacteria spots seen. Starting to grow thicker and more visible bacteria. |
|
|
Day 4 |
More small-big, white-grey bacteria spots seen. One or two spots are green. |
|
|
Day 5 |
Some more small-big white-grey bacteria spots seen. This time, some are green. Bacteria culture starting to form in green color. |
|
|
Day 6 |
More thick, small spots of bacteria seen in more rich-green color. Almost eery spot is green, and a little bit of small white spots cluster seen at some parts of the petri dish. |
|
|
Day 7 |
Not much difference from yesterday. More thick, small spots of bacteria seen in more rich-green color. Almost every spot is green, and a little bit of small white spots cluster seen at some parts of the petri dish. |
Bacteria growth (Gram-Negative bacilli) observations:
|
Bacteria: |
Days #: |
Qualitative Observations: |
|
2 Kitchen Sinks (Re-growth) |
Day 1 |
Very small, white-grey spots seen. Possible air bubbles in the liquid agar. |
|
Day 2 |
Aggressive signs of bacterial growth seen. Many small, white spots in dense bunches. |
|
|
Day 3 |
Again, more aggressive signs of bacterial growth seen. Dense, small white spots seen in the cluster formation the cotton swab was rubbed against on the agar. Other than looking a little denser, no other changes. |
|
|
Day 4 |
Aggressive white/grey tiny spots of bacteria. In cluster formation in the way the swab was swabbed on petri dish. |
|
|
Day 5 |
Aggressive white/grey tiny spots of bacteria. In cluster formation in the way the swab was swabbed on petri dish. A little denser and a little darker tint of grey-white than Day 4. |
|
|
Day 6 |
Not much difference from Day 5. Only a few more spots of bacteria seen and in the cluster formation. |
|
|
Day 7 |
Not much difference from Day 6. Only a few more spots of bacteria seen and in the cluster formation. |
Gram-stained bacteria under microscope:
|
Observations of microscope bacteria after gram-staining |
Kitchen counter and skin (Gram-positive) |
Very faint light red/pink. Lots of blots of purple seen. This is the skin bacteria. Meandering, longer lines seen. Microscope not powerful enough to see all bacteria cells. This is gram-positive bacteria. |
|
2 Kitchen Sinks (Gram negative) |
No faint purples. All light orange/pink and some small occasional blots of red. Small, black outlined lines seen. Could be bacteria. Microscope not powerful enough to see all bacteria cells. This is gram-negative bacteria. |
Results/Raw data:
|
|
Trials |
Trial 1 (diameter of inhibition in cm) |
Trial 2 (diameter of inhibition in cm) |
Trial 3 (diameter of inhibition in cm) |
Controlled |
|
Gram-positive bacilli (Bacterial growth inhibition in (cm)) |
Penicillin |
1.5 cm |
1.3 cm |
1.4 cm |
None |
|
Ampicillin |
0.9 cm |
1.0 cm |
1.1 cm |
None |
|
|
Neomycin |
0.0 cm |
0.0 cm |
0.1 cm |
None |
|
|
Erythromycin |
1.1 cm |
0.9 cm |
1.0 cm |
None |
|
|
Gram-negative bacilli (Bacterial growth inhibition in (cm)) |
Penicillin |
0.0 cm |
0.0 cm |
0.0 cm |
None |
|
Ampicillin |
0.6 cm |
0.7 cm |
1.2 cm |
None |
|
|
Neomycin |
1.2 cm |
1.0 cm |
1.1 cm |
None |
|
|
Erythromycin |
0.9 cm |
0.8 cm |
1.0 cm |
None |
Analysis
Analyzing results:
The results match my prediction/hypothesis and research about what bacteria each antibiotic targets. The effects of the antibiotics changed as the type of bacteria changed:
-
Penicillin's effectiveness/effects changed when comparing the gram-positive bacteria with the gram-negative bacteria. It was better with the gram-positive, seen with the graph. The average inhibition for gram-positive was 1.4 cm, and 0 cm with gram-negative.
-
Ampicillin was relatively similar with both bacilli types, but slightly more efficient with gram-positive by 0.17 cm—proves how gram-positive is easier to kill with its cell membrane, further proving the hypothesis, as seen by the graph. It is an average of 1 cm with gram-positive, and 0.83 cm with gram-negative.
-
Neomycin was much more efficient with gram-negative; this was the bacteria it was designed to target, supporting the hypothesis—antibiotics working with designed pathogens. It was 0.03 cm with gram-positive, and 1.1 cm with gram-negative.
-
Erythromycin, like ampicillin, had relatively similar effects with both bacilli, but was more efficient with gram-positive, when seen with the graph. It had an average inhibition of 1 cm with gram-positive, and 0.9 cm with gram-negative.
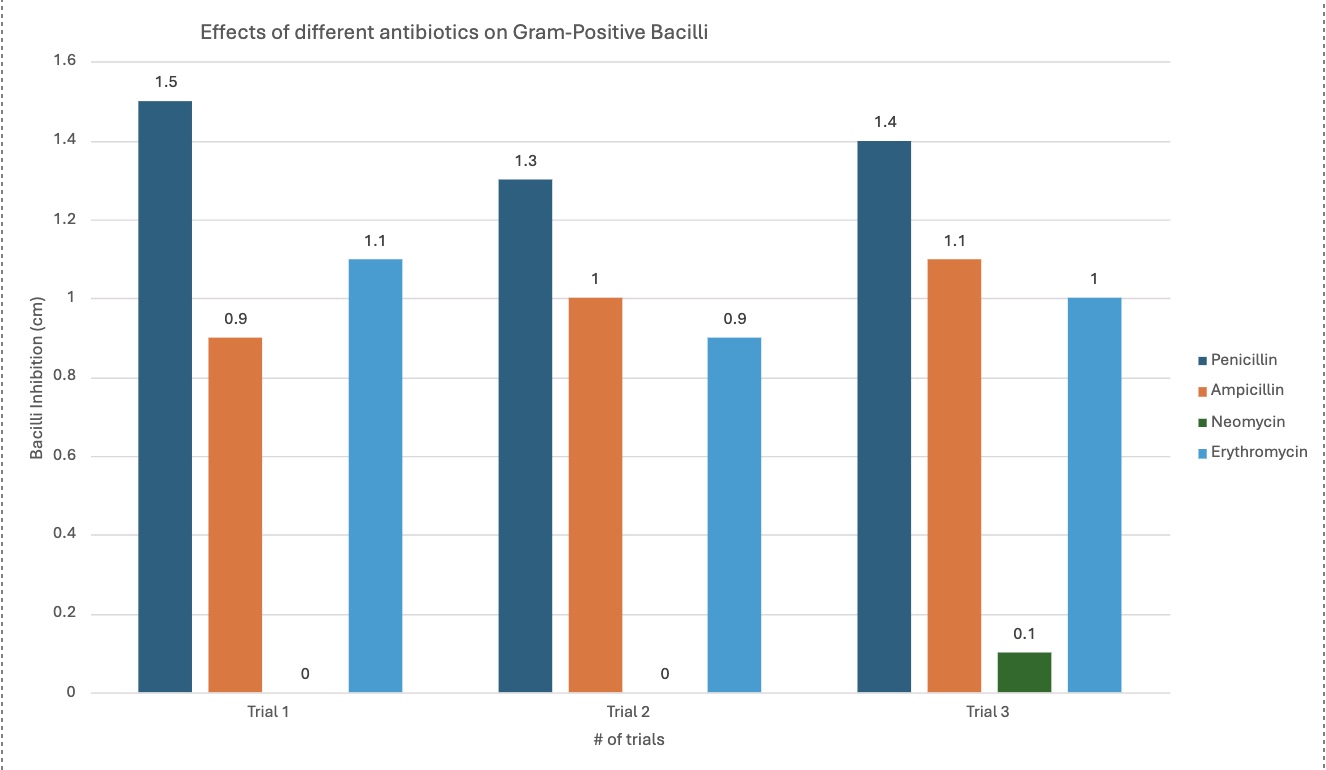
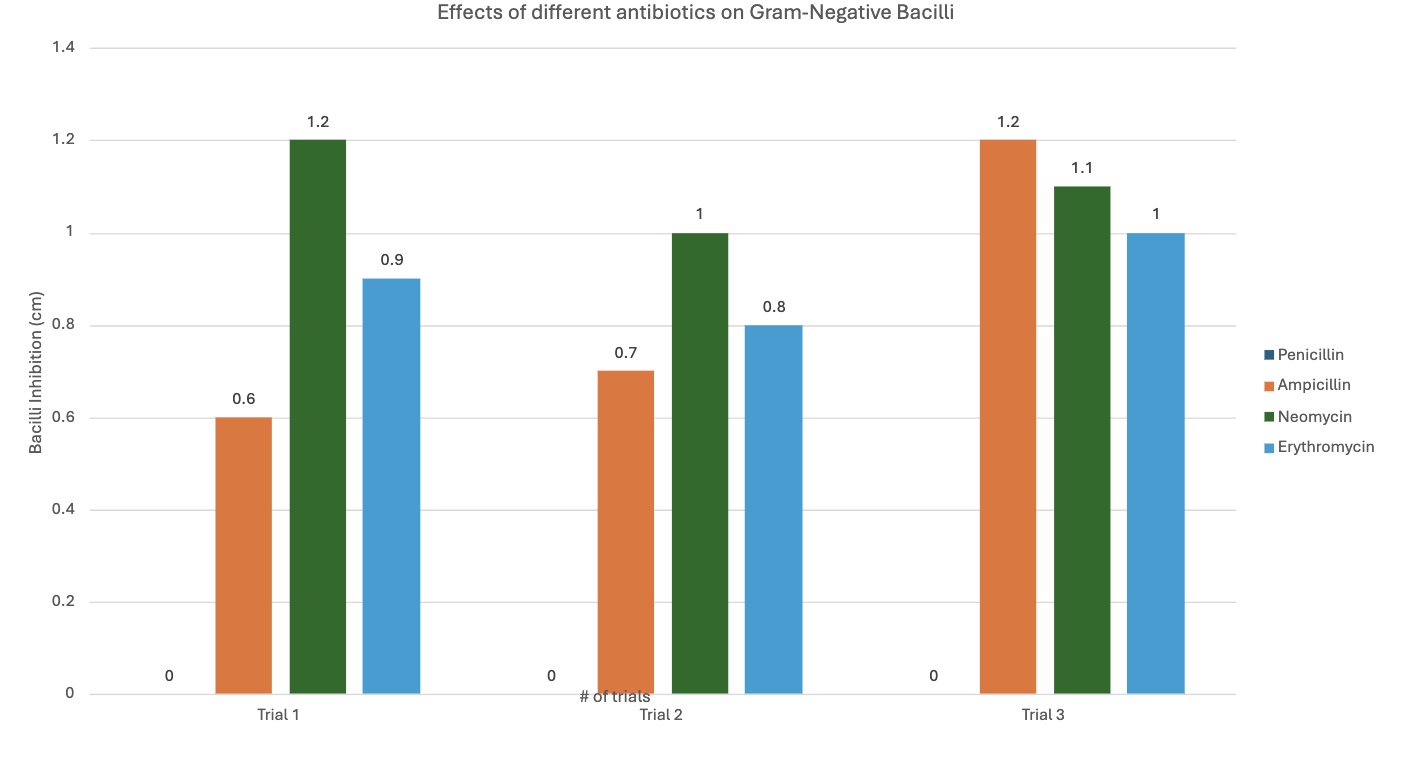
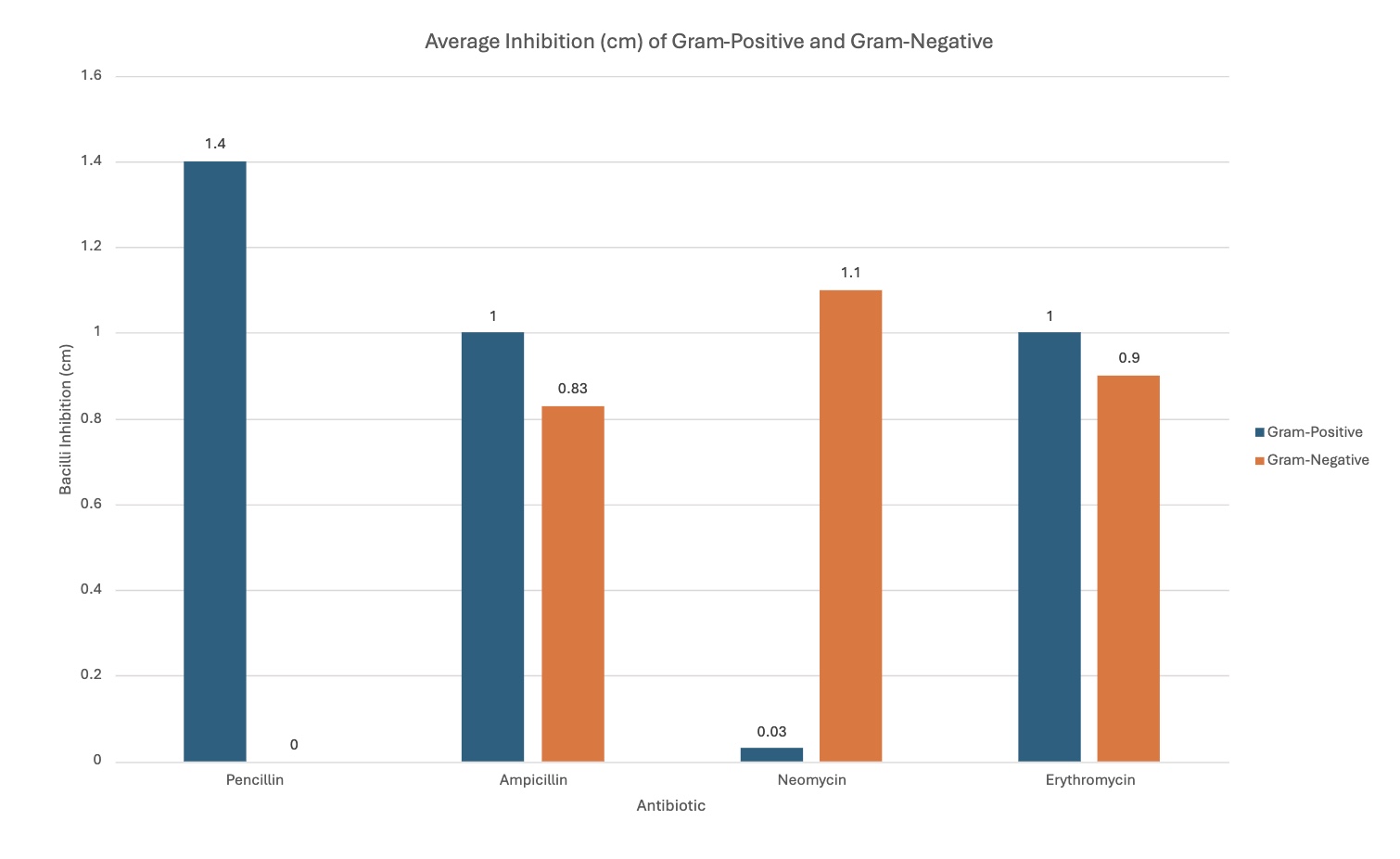
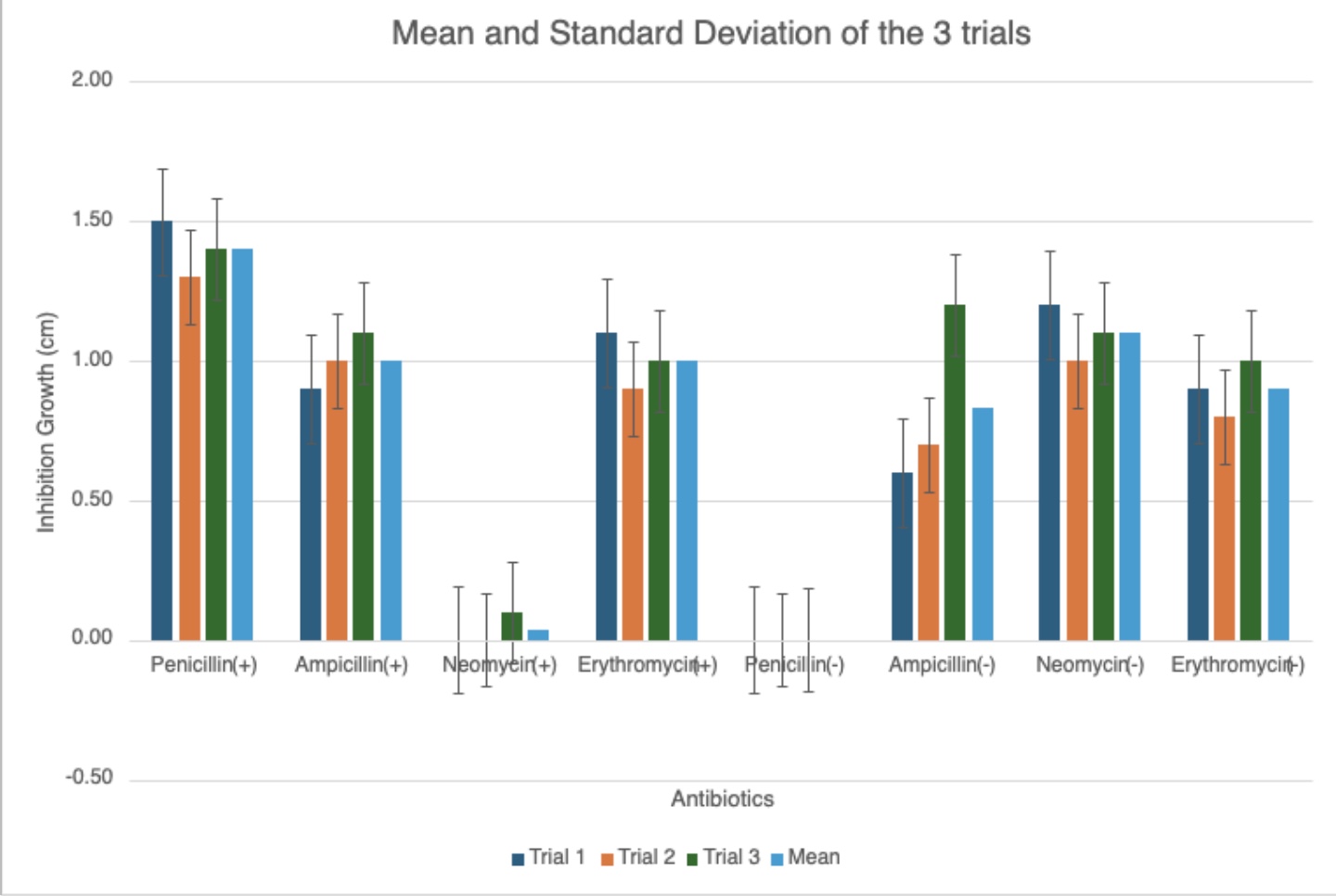
The different graphs show my raw data in different, compelling ways. Some graphs compare the trials for each antibiotic for each type of bacilli, showing differences between trials. Another graph compares the average inhibition between gram-negative and gram-positive bacteria for each antibiotic type, showing comparison between the kinds of bacteria, letting us see which bacilli an antibiotic was best against. The standard deviation graph utilizes error bars to show the fluctuations between trials, showing the uncertainty in my data and that there may be a few mistakes in measuring. The bigger the error bars, the more variation in data there is, showing with data sets may have more sources of error.
Closely analyzing the average inhibition between those antibiotics designed to kill both kinds of bacteria, it is clear that they were still best against the gram-positive bacteria. This also supports the conclusion/research how gram-positive bacilli have a single, thick cell membrane that easily absorbs substances, making it easier to kill, while the opposite applies to gram-negative.
Conclusion
The goal of the experiment was to see and solve the problem if the effects of antibiotics are identical on both gram-positive and gram-negative bacteria and prove it with different antibiotics and several trials to achieve more accurate conclusions. Looking at the raw data and graphs, the hypothesis was correct; the effects of antibiotics did change as the bacteria they were tested on changed. Evidenced with the graphs, specifically the mean and standard deviation graph, if the bacteria being tested on changes, the effects of the antibiotics (the inhibition growth) changes. This is because different antibiotics were engineered to target specific kinds of pathogens, making their effects unpredictable/different with non-targeted bacteria. Seeing the difference in inhibition the antibiotics had that were designed for both kinds of bacteria, they were still better with the gram-positive bacilli, also further proving the conclusion that gram-positive bacteria is easier to kill due to its cell membrane properties: having a single, thick cell membrane that easily absorbs substances. The experiment overall was successful, with raw data and graphs aligning with research, and the experiment data proving the hypothesis and presenting an accurate solution to the problem.
Application
Some future applications to the project include:
- Our modern healthcare is the future application to gram-staining and antibiotic testing. These processes are old practices used by scientists to tell which kinds of bacteria are causing what kinds of bacterial infections, and which medications are best effective against which. Thanks to these practices performed, they made a strong foundation to our currently reliable healthcare that provides antibiotics and prescriptions for people who really need them.
- In the future, if technology for these processes are developed and fail, we can still resort to these old practices to help deliver effective medications to those who need it.
Future research/possible applications to the project:
- We could search for more effective gram-staining methods beause the chemicals needed can be harmful and it takes a long time. We could possibly find technological solutions that are in-line with our current, modernizing world to find faster ways to see what kind of bacteria is causing which infections.
- Discover more effective antibiotic production methods; currently, they take very long to produce. We may integrate it with AI or some other technology to fight the most effective methods to quickly produce antibiotics for people who have serious bacterial infections.
Sources Of Error
Sources of error/limitations to the project:
-
Temperature. Could not control temperature (controlled variable) well. Optimal temperature to encourage bacterial growth could not be achieved. Bacilli grew at lower concentrations and slower speeds. Could not control due to the crazy, fluctuating winter season.
-
Gram-staining temperature. While gram-staining, the environment was very cold, which killed the first round of bacilli as well. The gram-staining environment temperature may have affected accurate gram-stained bacilli observations, explaining some unwanted colors.
-
Antibiotic inhibition measurement. Inadequate inoculation of bacteria for antibiotic testing, making it harder to see more accurate measuring. This is due to optimal temperature not being able to be maintained for bacterial growth, making less bacteria to be distributed between repitition of tests.
-
Bacilli under microscope after gram-staining could only be seen in blurs of colors. This may be a slight difference/inaccurate in observations section.
Possible improvements to project:
-
Let bacteria grow in an area where temperature is stable. Possible buy or create a small area such like that. to maintain optimal temperature and encourage bacilli growth.
-
Do gram-staining in an indoor area that is ventilated and safe instead of the garage. Maybe an unused bedroom so both bacteria can be safe and gram-staining observations could be a little more clearer, leading to more accurate observations.
-
Use a more efficient inoculating loop. So hard to use that I ripped agar off instead of just bacteria. Possibly watch tutorials to use properly or buy a more efficient one. This may also contribute to more inadequate innoculation of bacteria.
-
Use, rent, or borrow a more powerful microscope to properly see the bacilli. Or watch tutorials to use the current microscope to its full potential. This may also contribute to seeing the bacteria to a clearer magnification and make more accurate observations on the bacteria under the microscope.
Citations
-
Janessa Bretner. (2023, September 8). STEMIA Group Materials. https://stemia.schoology.com/group/6912913417/materials#/group/6912913417/materials?f=312138664
-
Home Science Tools Learning Center. (2024, March 11). Gram Stain Antibiotics Project. https://learning-center.homesciencetools.com/article/gram-stain-antibiotics-project/
-
WebMD Editorial Contributers. (2024, February 20). What Are Antibiotics? https://www.webmd.com/a-to-z-guides/what-are-antibiotics
-
Karen Steward, PHD. (2019, August 21). Gram-Positive vs. Gram-Negative. https://www.technologynetworks.com/immunology/articles/gram-positive-vs-gram-negative-323007
-
Wiktionary. (2024, March 11). monoderm. https://en.wiktionary.org/wiki/monoderm#:~:text=monoderm%20(not%20comparable),a%20thick%20layer%20of%20peptidoglycan
-
Google Search. (2024, March 11). How to Grow Actual Bacteria Culture. https://www.google.com/search?q=how+to+grow+actual+bacteria+culture&sca_esv=580369605&tbm=vid&source=lnms&sa=X&ved=2ahUKEwibzNPWwbOCAxXNCTQIHcmmB8cQ_AUoAnoECAUQBA&biw=1470&bih=764&dpr=2#fpstate=ive&vld=cid:59917b5c,vid:Bwjxi3vS0K4,st:0
-
Home Science Tools Learning Center. (2024, March 11). Bacteria Experiment Guide. https://learning-center.homesciencetools.com/article/bacteria-experiment-guide/
-
WebMD Editorial Contributers. (2023, May 23). Difference Between Gram-Positive Bacillus & Gram-Negative Bacillus. https://www.webmd.com/a-to-z-guides/difference-between-gram-positive-bacillus-gram-negative-bacillus
-
US Micro Solutions. (2024, March 11). Gram-Negative Bacteria. https://www.usmslab.com/gram-negative-bacteria/#:~:text=In%20ISO%2Dclassified%20areas%2C%20the,other%20sources%20of%20standing%20water
-
Kirsten Nunez. (2019, December 18). Gram Positive Bacteria Explained In Simple Terms. https://www.healthline.com/health/gram-positive#:~:text=Most%20of%20these%20bacteria%20are,can%20cause%20serious%20medical%20conditions
-
HealthyChildren.org. (2024, March 11). How Do Antibiotics Work? https://www.healthychildren.org/English/health-issues/conditions/treatments/Pages/How-Do-Antibiotics Work.aspx#:~:text=Some%2C%20such%20as%20penicillin%2C%20kill,nutrients%20they%20need%20to%20survive
-
Google Search. (2024, March 11). Bacilli. https://www.google.com/searchsca_esv=582393838&sxsrf=AM9HkKmw2BVLhWvz7qUD-CDK739dIjjnLg:1699996264443&q=bacilli&si=ALGXSlZCBshTM3a3nPTSW0d1OmQeLsPCMAUICQClim3BPnviAcvMht1lQi2kqobyIs7YZ1lay7Us0csS1_Wes3FVrR6wj7GE6w%3D%3D&expnd=1&sa=X&sqi=2&ved=2ahUKEwj6q9jLs8SCAxUYMTQIHb1SAYQQ2v4IegQIGhAR&biw=1633&bih=892&dpr=1.8https://www.google.com/search?sca_esv=582393838&sxsrf=AM9HkKmw2BVLhWvz7qUD-CD
Acknowledgement
Credits:
1) Sriram Dokuparti – helped at several parts of the project such as helping record data while I measured.
2) Friends and family – helped provide advice for several parts of the project, such as choosing the topic.
3) Teacher (Science Fair Lead) - helped be with advice at every single doubt I had (could not have done without).
4) HomeScienceTools – gave me tools I needed last minute which I could not find anywhere else.
5) Google - main search engine. Let me get sources and information I needed to get research and do my project.