NeuroPulse: A Haptic Feedback Platform for the Treatment of Movement Disorders, Epilepsy, and Sports Injuries
Grade 11
Presentation
No video provided
Hypothesis
Experiment 1, Testing the Effect of NeuroPulse on Active Inhibition: NeuroPulse increases the difference between arm angles compared to the control difference between the angles of the arms, suggesting that it is an effective activator of active inhibition mechanisms.
Experiment 2, Testing the Effect of NeuroPulse on Grip Strength: Haptic feedback reduces grip strength, suggesting that it could modulate muscle activity in movement disorders and seizures by reducing the strength of muscle contractions.
Research
Haptic feedback (e.g buzzes and clicks) as a therapy, though untested, has a variety of potential application in epilepsy, movement disorders, and sports injuries. For example, It is thought that epilepsy is caused by excessive electrical discharges in certain parts of the brain. But how do seizures stop? In Dynamics of muscle activation during tonic-clonic seizures, Conradsen et al. recorded electromyography of the deltoid muscle during a generalized tonic-clonic seizure (GTCS), which is a type of seizure. In the study, where they used muscle activity as a surrogate for brain activity, they suggest that “that the same inhibitory mechanisms that contribute to GTCS termination counteract seizure initiation” and that “both active inhibition and mechanisms related to metabolic depletion act synergistically to stop the seizure.” Active inhibition is effectively attempting to actively inhibit a physiological process; some of the ways that this system can be activated is by sensations of stretch and muscle resistance, which actively inhibit behavior.
Those sensations are the most accessible active inhibition system, and together with other mechanisms they are know as proprioception, or kinaesthesia, which is the sensory system that tells “you what is being done to your body and what your body is doing” (Taylor, 2013). Through muscle spindles, golgi tendon organs (GTOs), and skin mechanoreceptors, this system perceives physically where your body is in space, how much stretch or resistance your muscles are under, and how much pressure there is on your skin. Through haptic feedback, we can manipulate this system to create illusory sensations that have real clinical impact. In the case of epilepsy, kinaesthesia can be manipulated to cause different sensations that may result in reduction of seizure duration, frequency, and severity, as Conradsen et al. seem to suggest.
One of the advantages of haptic feedback over vibration therapy is that haptic actuators, things that produce haptic feedback, are easily programmable and wearable, resulting in something that is as portable and effective as a pacemaker but noninvasive. Its programmable characteristics allow sequences of haptic feedback to be personalized for patients, and its wearability allows it to treat disorders that could otherwise not be treated.
Dystonia is a movement disorder that involves “sustained involuntary muscle contractions with twisting, repetitive movements” (Movement Disorders - Symptoms and Causes - Mayo Clinic, 2022). If dystonia functions similarly to epilepsy in that active inhibition mechanisms end and reduces episode chance, haptic feedback may help. Furthermore, there is some evidence that vibration therapy may reduce the strength of muscle contractions in general, suggesting that, when haptic feedback involves vibration, it may reduce the strength of contractions (Lupowitz, 2022).
It is through this mechanism that haptic feedback may help physiotherapists treat sports injuries and other maladies. Vibration therapy is proven to relieve pain and help throughout the healing process.
The engineering of this technology, NeuroPulse, will be covered in the application section of this project. The basic goals of this project are to create a tool that clinicians and patients can use to activate active inhibition mechanisms and treat illnesses using vibration therapy and to test this technology to see if it is clinically feasible.
Variables
Experiment 1:
- Manipulated Variable: Haptic Feedback on Biceps tendon
- Responding Variable: Angle of Arms
- Controlled Variable: Participant
Experiment 2:
- Manipulated Variable: Haptic Feedback on flexor digitorum profundus muscle
- Responding Variable: Grip Strength
- Controlled Variable: Participant
Procedure
Procedure 1: Testing the Effect of NeuroPulse on Active Inhibition
Materials:
- Blindfold
- Cushions for elbow support
- Large Protractor or angle measuring device
- Haptic feedback apparatus
- Timer
Objective: To assess whether haptic feedback buzzing can effectively stimulate proprioceptive responses and create kinesthetic illusions.
Hypothesis: Haptic feedback increases the difference between arm angles compared to the control difference between the angles of the arms, suggesting that it is an effective activator of active inhibition mechanisms.
Null hypothesis: There is no difference in the differences between the angles in the haptic feedback and control groups, suggesting that haptic feedback is not an effective activator of haptic feedback.
This experiment is adapted from USYD Physiology, 2020.
- Seat the participant comfortably with arms extended and elbows resting on two cushions. There should be a large protractor between the participant’s arms.
- With the participant’s consent, place the blindfold on the participant. A blindfold needs to be placed in order to eliminate visual cues; the participant needs to go purely off proprioception for this experiment.
- With the participant’s consent, place the Velcro strap with the haptic actuator on the musculotendinous area of the bicep of the participant’s dominant arm.
- Ask the participant to raise their nondominant arm to a 30 degree angle. Using the large protractor and with their consent, gently correct their arm to a 30 degree angle. This will give them an idea of what a 30 degree angle looks like.
- Ask the participant to raise their dominant arm to match their nondominant arm. Measure the angle, and compute the angle difference between the arms. Remove the blindfold and ask, on a scale of 1 -10, about the perceived angle of each arm vs the actual angle of each arm, where 1 is no difference and 10 is extreme difference.
- Ensure that the actuator is placed on the subject’s bicep comfortably, properly, and that they can feel the actuator buzzing during a test of it.
- With the participant’s consent, place the blindfold on them again.
- Repeat the arm-raising procedure, but activate the haptic actuator 5 seconds before and during the dominant arm raise. Notify the participant of these times, and always turn it on with their consent.
- Measure the angle of the right arms again and calculate the difference in angle between each arm. Repeat the qualitative test regarding arm difference.
In terms of data analysis, a significance test and a t-test will be conducted on the quantitative data to determine significance. The qualitative data will be analyzed as well to draw a conclusion from the data.
Experiment 2: Effect of NeuroPulse on Grip Strength
Materials:
- Grip trainer
- Haptic Feedback apparatus
- Timer
- Blindfold
Objective: To investigate the impact of prolonged, buzzing haptic feedback on grip strength.
Hypothesis: Haptic feedback reduces grip strength, suggesting that it could modulate muscle activity in movement disorders and seizures by reducing the strength of muscle contractions.
Null Hypothesis: Haptic feedback has no effect on grip strength, suggesting that it is not an effective treatment modality for movement disorders and epilepsy.
Procedure:
- With the subject’s consent, place the Velcro strap on the subject’s forearm over the Flexor digitorum profundus muscle without activating the haptic actuator. This muscle is one of the main muscles responsible for grip strength, so it works well with the aims of the project.
- With their consent, the subject will also be blindfolded in this experiment so that they do not modulate their strength based on sight.
- With the subject’s consent, instruct the participant to squeeze the grip trainer with approximately 50% strength for 10 seconds.
- Measure and record the displacement of the grip trainer handles.
- If the subject wants to, they can take a “blindfold break” and take it off for a moment. The blindfold should be on for this experiment, however.
- Ensure that the actuator is placed on the subject’s Flexor digitorum profundus muscle comfortably, properly, and that they can feel the actuator buzzing during a test of it.
- With the subject’s consent, turn on the actuator 60 seconds before the grip action.
- Ask the participant to perform the grip action as in the control experiment, but turn off the vibration during the contraction.
- Measure and record the displacement of the grip trainer handles.
In terms of data analysis, we could take the raw displacement scores as corollaries of muscle force. We would expect to see decreased grip strength as an outcome that would prove the hypothesis correct.
Debriefing
- Subjects will be briefed on the results of their experiment orally. If they request a written copy of the results, it will be provided to them.
Observations
Experiment 1:
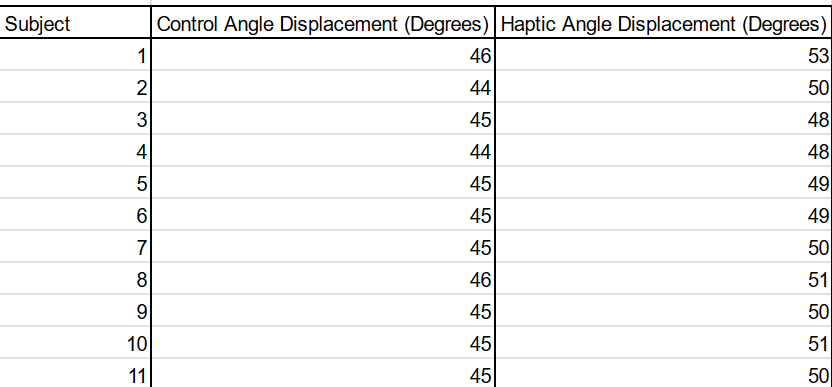
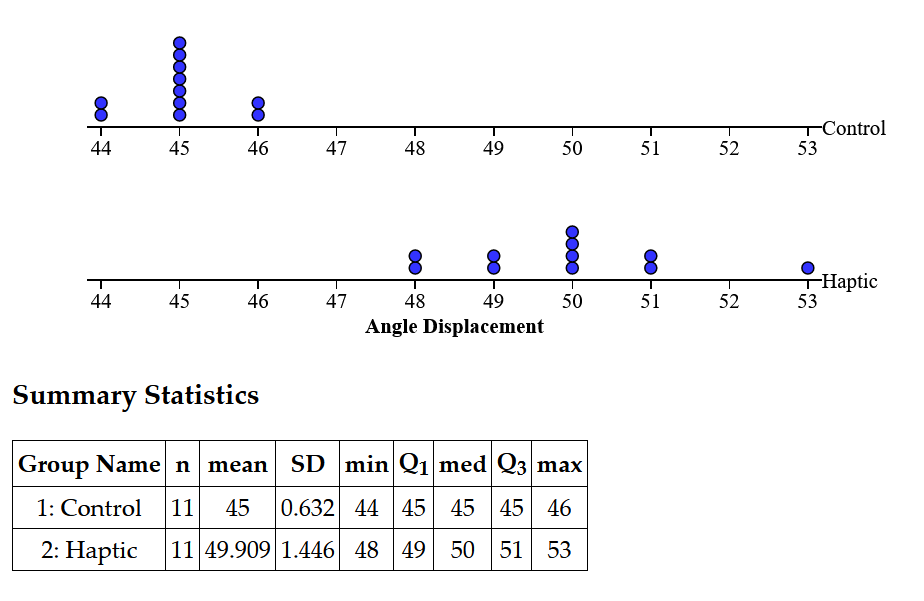
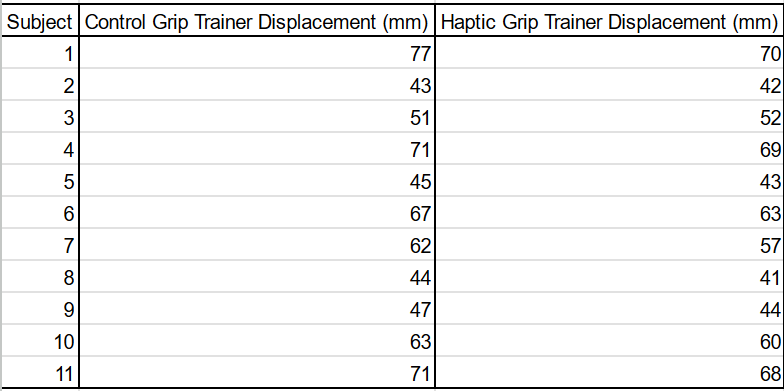
Experiment 2:
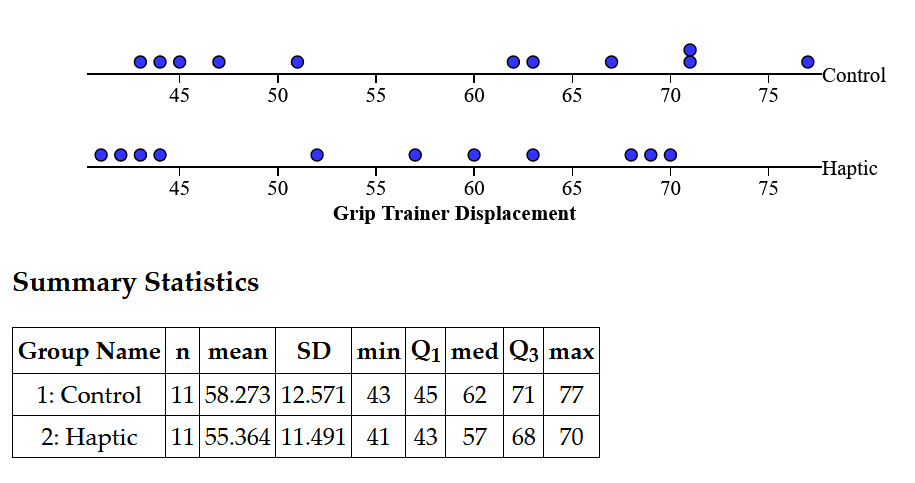
Analysis
Experiment 1:
For this experiment, since the distribution was normal as determined by a probability plot, a one-tailed paired T-test was conducted between the two treatments. The p-value was found to be p < 0.000000027, suggesting high statistical significance. This means that the null hypothesis can be rejected as it was clearly shown that the Haptic Feedback treatment increased the displacement between the arms in the blindfolded angle matching task compared to the control by an average of 4.9 degrees.
Experiment 2:
For this experiment, the distribution was bimodal, likely due to gender differences in arm strength. This means that it could not be analyzed by T-tests or Chi-Squared tests to determine statistical significance since it was not normal. The median of the haptic feedback treatment was five mm lower than the median for the control group, perhaps suggesting some effect, but the results are too varied to tell for certain. From a graphical perspective, the haptic feedback group shows lower values, which agrees with the hypothesis, but this is confounded by high variability in the dataset. For now, there is not enough evidence to reject the null hypothesis.
Conclusion
In conclusion, there are three ways that this experiment contributes to the scientific and medical enterprise:
-
Suggests that active inhibtion and vibration therapy are therapeutic targets for epilepsy, movement disorders, and sports injuries
-
Proves that haptic feedback activates active inhibition mechanisms, thus leading to possible treatments for epilepsy and movement disorders
-
Establishes NeuroPulse as a feasible therapeutic target for these illnesses
The next steps for this project would be to do further testing in order to test NeuroPulse as vibration therapy, which would definitively make it an extremely promising therapeutic target for sports injuries, test the limits of the effects of NeuroPulse using a battery of neurological tests, possibly revealing other therapeutic targets, and improve the product even further.
Application
Engineering:
NeuroPulse is composed of the following components First, a microcontroller (Arduino Uno S3) will be used to write a script of alternating clicks and vibrations. This will be connected to a DRV8662 EVM, which drives the electricity to higher voltages, which are necessary to get the actuator to work. A piezoelectric actuator (9mm PowerHap) will be used for the actual haptic feedback, since it can generate arbitrary waveforms and has low latency. The actuator can also relay signals back to the microcontroller, making it ideal for further development of this project through the detection of seizures or movement disorder episodes.
NeuroPulse is easily programmable, meaning that clinicians can customize the pattern of vibration so that it can be modified to suit the patient. NeuroPulse also has the capacity to detect muscle contractions to an extent, though this feature is still a work in progress. This may eventually lead to the detection of seizures or movement disorder episodes. For more details on the engineering of the project and its applications, visit sites.google.com/view/neuropulse/home, where all information regarding the development of this project is available for free so that patients, caregivers, and clinicians, can access it.
Applications:
The experiments are very suggestive of success, connecting NeuroPulse to the main messaging pathway that contributes to seizure and movement disorder episode end: active inhibition. This mechanism could also be used to treat sports injuries as described below.
The first is the possibility of treating epilepsy/seizures using simple haptic feedback, or buzzers on the skin. This treatment would complement anticonvulsant medication and it could be personalized to the patient, similar to how doctors often change the pattern of deep-brain stimulation for better results for the patient. The success of this experiment would also open doors for more simple and accessible epilepsy treatments involving kinaesthesia.
It would prove that kinaesthesia, and the feedback associated with it, functions even during the extreme excitatory conditions of epilepsy, and therefore offers a treatment for movement disorders and awkward gait. This treatment could also be leveraged in physiotherapy; if a patient has an awkward gait, haptic feedback devices could be used to correct the gait. Underused muscles could also be strengthened through this method; one muscle could be inhibited, forcing the other muscle to work harder.
These applications would require precise haptic feedback and small haptic actuators. This is why the experiment leverages the emerging technology of piezoelectricity to do so. Piezo haptic actuators can be incredibly small and can generate arbitrary waveforms, allowing for precise, personalized treatment for the patient.
Sources Of Error
Experiment 1:
The main source of error with this experiment lies with the placement of the haptic feedback. Since this type of haptic feedback is weak and strong only in a certain area, it needed to be precisely placed on the biceps tendon to have its full effect. It sometimes took a little while to place the strap which contains the actuators that produce the feedback on the correct place. Small variations in this process may have contributed to inconsitent data. Although, from what it looks like, the data was extraordinarily consistent and this would have only had a minor effect.
Experiment 2:
In this experiment, we did not account for the difference in physical performance between participants during the design process. The data was bimodal, perhaps suggestive of the difference between physical performance between males and females. Within the two areas there was further variation, likely between more and less athletic males and females. These represent the two differences between groups that we need to control for in order to adequately determine whether NeuroPulse acts as vibration therapy. For future designs, we need to focus on a single gender with similar physical performances; for example, choosing only males who have participated in a sports team recently would reduce the variation and likely make grip strength a normal distribution which can then be analyzed properly. While further testing needs to be conducted, the information acquired in the experiment is promising for NeuroPulse.
Citations
Conradsen, I., Moldovan, M., Jennum, P., Wolf, P., Farina, D., & Beniczky, S. (2013). Dynamics of muscle activation during tonic–clonic seizures. Epilepsy Research, 104(1–2), 84–93. https://doi.org/10.1016/j.eplepsyres.2012.09.004
Flexor digitorum profundus. (n.d.). Physiopedia. https://www.physio-pedia.com/Flexor_Digitorum_Profundus
Lupowitz, L. (2022). Vibration Therapy – a clinical commentary. The International Journal of Sports Physical Therapy, 17(6). https://doi.org/10.26603/001c.36964
Movement disorders - Symptoms and causes - Mayo Clinic. (2022, May 24). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/movement-disorders/symptoms-causes/syc-20363893#:~:text=The%20term%20movement%20disorders%20refers,Ataxia.
Piatin, V., Shirolapov, I., & Nikitin, O. (2009). [Vibrational physical exercises as the rehabilitation in gerontology]. PubMed. https://pubmed.ncbi.nlm.nih.gov/19947400/
Pickar, J. G., & Wheeler, J. D. (2001). Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. Journal of Manipulative and Physiological Therapeutics, 24(1), 2–11. https://doi.org/10.1067/mmt.2001.112017
Psy, S. O. (2022, January 1). Mechanoreceptors. Pressbooks. https://pressbooks.umn.edu/sensationandperception/chapter/mechanoreceptors-draft/
Taylor, J. L. (2013). Kinesthetic inputs. In Springer eBooks (pp. 931–964). https://doi.org/10.1007/978-1-4614-1997-6_31
USYD Physiology. (2020, October 7). F) proprioception [Video]. YouTube. https://www.youtube.com/watch?v=DUeJHIpSwRA
Wang, Y., Wei, P., Feng, Y., Luo, Y., & Zhao, G. (2022). Animal Models of Epilepsy: A Phenotype-oriented review. Aging and Disease, 13(1), 215. https://doi.org/10.14336/ad.2021.0723
Acknowledgement
I would like to thank Dr. Nathalie Jette and Dr. Guillermo Delgado of the Calgary Comprehensive Epilepsy Program for all their help in developing the project. I would also like to thank our Science Fair Coordinator, Ms. Heather Lai, for her guidance and hard work to make CYSF available at our school.