Hydrogels: The key to saving water in agriculture
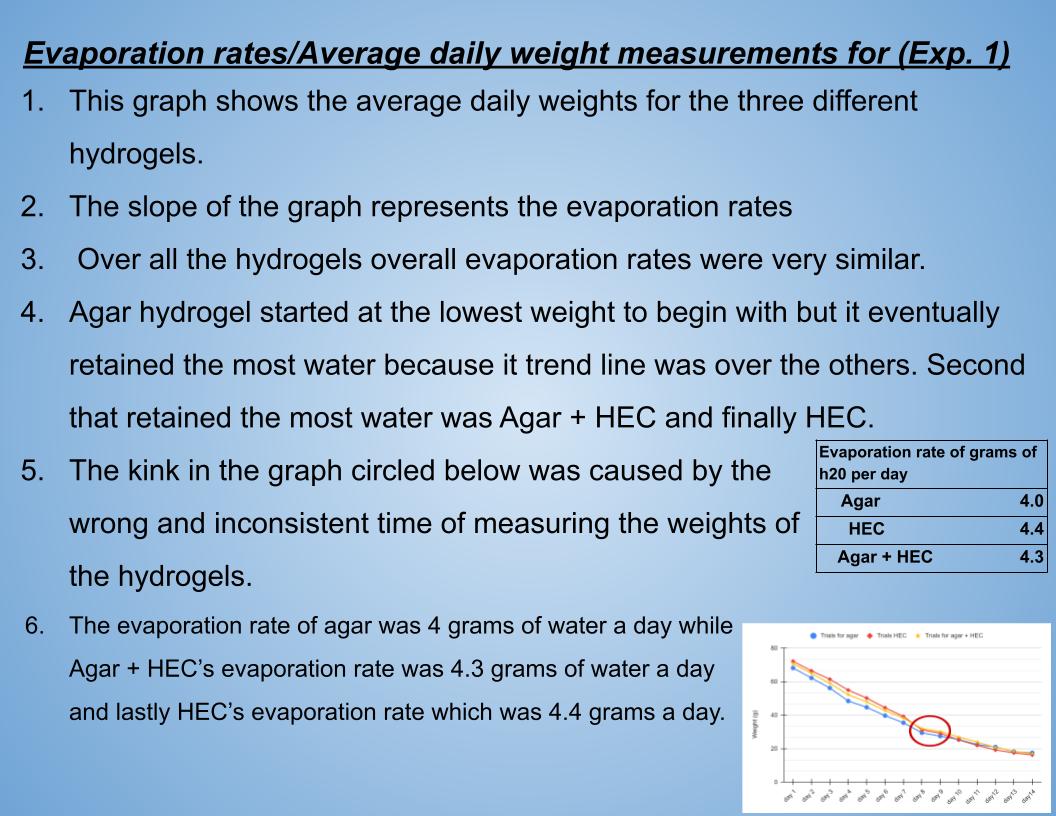
Grade 7
Presentation
Hypothesis
Hypothesis 1.0
If the hydrogels are placed in water, hydrogels absorb maximum water, and weights are recorded over 14 days, then the hydroxyethyl cellulose hydrogel will retain the most water overtime (over 14 days) because the strong repeating polymers made of glucose will trap the that water over time. Plus agar hydrogel can be reversible and turn into liquid with the cold temperature of the environment the experiment take place in, thus the agar hydrogel and HEC + Agar hydrogel will not hold the maximum or majority of its water over time.
Hypothesis 2.0
If three different hydrogels are placed in water and are given a chance to absorb water then agar hydrogel will absorb the most because agar contains special polymers that swell up to hold water as well as trapping it.
Research
What is a hydrogel? Hydrogels are a material that hold water really well. They contain of big molecules that are surrounded in smaller molecules that are laid together in a certain pattern to hold water molecules.These hydrophobic/hydrophilic molecules act like a cage and trap water, this is called a polymer. When the polymers meet it is called a “Cross link.”**
How are hydrogels used in our daily lives? They are used for contact lenses, hygiene products, and even wound dressings. They are also used for drug delivery and tissue engineering.
Can agar help with plant growth? “Research grade agar is used extensively in plant biology as it is optionally supplemented with a nutrient and/or vitamin mixture that allows for seedling germination in Petri dishes under sterile conditions (given that the seeds are sterilized as well).” -Wikipedia
Agar is meant to contain all the nutrients that the new cells or tissues need to grow and become seedlings.
Agar generally holds the following hormones to stimulate optimal development:
1. Cytokinins (these assist with stem growth and increase cell division)
2. Auxins (these help with new root growth and increase cell division)
How is it a possibility to use hydrogels to conserve water? Irrigating crop fields like this one bellow can take thousands of liters of water, a portion of which is evaporated from the unused water from the soil. Also, plants transpire approximately 95% - 98% of the water they suck up through their roots, this means that they use 5% - 2% of their water. This means that the hydrogels might slowly release the water, thus conserving the water and giving the plant what it needs. Or the roots can grow towards the hydrogel when they need water, acting like a fridge, so when the plant needs water, it can grow towards the hydrogel and take water when necessary. Lastly, the hydrogel may absorb water from the soil faster than the plant or for the water evaporating, thus it helps retain soil moisture. ⬅️ This is a theory.
What does H.E.C stand for? It stands for Hydroxyethyl Cellulose.
(This ingredient is one compound of my hydrogel.)
How can roots move toward water?
Roots can sense the moisture in the soil. They have specialized cells and tissues that can detect changes in water concentration. When the root detects a concentration of water it initiates a growth response. The cells on one side of the root grow longer, causing the root to curve towards the direction of higher water concentration.
What is the chemical compound for hydroxyethyl cellulose?
It is (C2H6O2)n Meaning two carbon atoms, six hydrogen atoms, and lastly two oxygen atoms. The “n” at the end means that there are many HEC molecules stuck together.
Why is hydroxyethyl cellulose a gelling agent?
When HEC is added to water, the hydroxyethyl groups interact with water molecules through hydrogen bonding. This interaction causes the polymer chains to spread out and become entangled in the water molecules. This structure thickens the water and gives it a gel-like consistency.
Why is agar a gelling agent? Hydrocolloid Property: Agar has a special property called hydrocolloid behavior. This means it can form a gel in the presence of water. Fact* Agar can also be transformed into plastic and used in many sauces. Gel Formation: When agar is heated in water, it dissolves to form a clear solution. However, when this solution cools down, agar molecules arrange themselves in a three-dimensional network. This network traps water molecules and forms a gel.
Variables
Variables
1. Manipulated: The different hydrogels
2. Responding:
-what hydrogel absorbs the most water
-What hydrogel retains the most water the longest
3. Control (for experiment 2): Container with just soil to measure evaporation rate.
(There wasn’t need to have one in experiment 1 because it was only needed to compare the data to the others)
4. Constants: Same container, same potting soil,same temperature of boiling water, same room temperature, same scale, same water, same measuring cup, and same cup that hydrogels were mixed in.
Procedure
Materials
- Kitchen scale
- Agar powder, (60 grams).
- Hydroxyethyl cellulose (HEC) powder, (60 grams)
- Citric acid, (30 grams).
- Water.
- Kettle or device for boiling and easily pouring water
- 4-cup (1-liter) Pyrex measuring cup or other similar sized heat-resistant container
- Spoon
- Fork
- 90 x 15 mm petri dishes or small containers(30) [8oz]
- Paper towel
- Containers or bowls than can hold at least 1 cup of water (12)
- Potting soil, preferably without (water-holding agents);
- Small pots (16); seedling pots work well
- Measuring cup or gradual cylinder, 60ml
Procedure phase 1 (creating the hydrogels)
- Gather materials
- Label containers for which hydrogel will go in there
- First start by making the Agar hydrogel:
- Get 40 grams of agar powder and 10 grams of citric acid and mix the dry ingredients in the heat resistant measuring cup.
- Next boil more than 500ML water in a kettle to (100℃)
- Pour 500ml of the boiled water into the cup and start mixing until it’s very thick or hard to stir.
- When all citric acid and agar powder clumps are gone carefully pour 60 ml of hydrogel into small container, or tare container and pour 60 grams of hydrogel.
5. Second make the HEC Hydrogel:
- Repeat same method for the agar hydrogel except instead of using 40 grams of agar, use 40 grams of hydroxyethyl cellulose powder and 10 grams of citric acid.
6. Third make the Agar and HEC hydrogel:
- Mix 20 grams of Agar powder with 20 grams of Hydroxyethyl cellulose in a heat resistant measuring cup
- Next boil more than 500ML water in a kettle to (100℃)
- Pour 500ml of the boiled water into the cup and start mixing until it’s very thick or hard to stir.
- When all citric acid and agar powder clumps are gone carefully pour 60 ml of hydrogel into small container, or tare container and pour 60 grams of hydrogel (pour 60 ml of hydrogel in each of the special containers for sub experiment #2 and 60 ml )
7. Let hydrogels cool for 3 hrs, but do not leave them for more than 48 hrs (put lids on them).
Procedure phase 2 (how much can the hydrogel absorb and retain over time)
- After hydrogels are cooled, label containers.
- Add 60 ml of water to all hydrogel container
- Weigh hydrogel in container
- Wait an hour for hydrogel to reach maximum absorption
- Weigh hydrogel in container and subtract initial weight of container.
- Enter data into spreadsheet
- Then for 14 days record observations and and weight of hydrogel.
Procedure phase 3 (How much can the hydrogels retain in soil)
- Use fork or spoon and cut hydrogels into small pieces (the size of a pea)
- Add 50 grams of potting soil to container
- Then add 50 ml of water
- Weigh container
- Record weights and observations of containers for 14 days
Observations
Qualitative observations for procedure phase 1
1. Agar: While making this hydrogel it was hard to mix as their were lots of big clumps of the dry ingredients. When making this hydrogel it smelled really bad sort of like a very dirty or unclean pool. 15 minutes after I poured the hydrogels I notice that is was a light yellow in colouration and it was the most opaque and I couldn't see through it at all. It settled in a lump
2. Hydroxyethyl cellulose: While making this hydrogel it was really easy to mix and lumps of the dry ingredients quickly mixed with the water. This hydrogel smelled like vinegar or some type of acid. 10-20 minutes later after I poured the hydrogel into the container I noticed it had lots of small bubbles near the surface and compared to the others it was really translucent and it settled all around the container
3. Agar + HEC: While making this hydrogel it was fairly easy to mix but was a little harder that HEC. There were some lumps from the dry ingredients but it was easy to mix.

Day 0
Hec: when soaking the HEC hydrogel in the 45th minute there was some puffy bits of hydrogel starting to expand outward. HEC hydrogel looked that it was swelling the most out of all the hydrogels. Also HEC hydrogel had lots of tiny bubbles. While doing experiment 2 and cutting the HEC hydrogel into pieces the hydrogel was very jiggly and felt a lot like JELLO™️.
Agar: Agar hydrogel looked like it didn’t absorb much water but over time I noticed that the water sitting on top of the hydrogel was less and less visible over time. It still had a stinky smell like in procedure phase 1. In experiment 2 it felt like a really firm fruit sort of like a ripe mango. It was the easiest to cut into pieces.
Agar + HEC: When soaking the hydrogel before and after it felt a little different as the hydrogel was less sticky at the start but became goopy and very sticky at the end. Just like the agar hydrogel it was hard to see major absorption. This hydrogel still had white dot/clumps of white in the hydrogel.

Day 1-2
HEC: This hydrogel still had bubbles
Agar: In experiment 1 the hydrogel hardened significantly but not to the extent that it was super hard that it wasn't retaining moisture. Later I realized that the was hard layer around the outside but it was still moist and wet on the inside.
Agar + HEC: This hydrogel was still sticky and goopy but unlike agar and HEC this hydrogel did not settle and all the hydrogel was not touching around the container. White dots were still prominent throughout the hydrogel.
Day 3-7
Mold growth: On day 3 there was a little bit of mold growth on every container in experiment 2 but no mold growth on trials for Agar + HEC. The place with the most mold growth was agar trial 3 and 4.
HEC: this hydrogel lost the majority of the water on top. No noticeable changes other than the hydrogel was only a little bit dry and hard.
Agar: Was now very hard and not that squishy anymore
Agar + HEC: This hydrogel was not sticky anymore but it felt like agar at the start of the experiment.

Day 8-9
The soil was fair and dry
First day that agar trial 1 (experiment 1) was dried and shriveled up and it light brown.
On day 9 Measurements were missed, so measurement were done in the early morning.
Mold growth: There was significant more amount of mold growth on day 9 compared to day 3

Day 10
All hydrogels are shrinking and HEC in peeled off of container (trial 3, 4)
Hec has water and dark color at bottom of container, also very rigid.
Agar is brown and size of finger.

Day 11
Agar: agar trial one (experiment 1) is rock hard and others are starting to solidify. Based on the data agar trial 1 evaporated all of its water (potential)
HEC: Hec trial 1 (Experiment 1) is the hardest out of them all. Hec for experiment 2, all the containers have lots of water at the bottom
Agar + HEC: in experiment 1 all of them are slightly squishy and still have prominent white dots in them.
Day 12-14
Agar + HEC: From day 12-14 this hydrogel had large white dots that were very hard. Overall every day there was noticeable shrinking every day. Also compared to agar its color still stayed the same with only a very small amount of the hydrogel changing colour.
HEC: Hec had the smallest change in appearance as it did not change colour over days 12-14 and only 1 hydrogel decreased in their size.
Agar: Agar’s physical appearance changed the most, as it was very noticeable to see the different colours and size throughout day 12-14.
All hydrogels with a hydrogel that was measured incorrectly and was significantly smaller than the rest of the hydrogels in its group were the darkest throughout the experiment.

Analysis
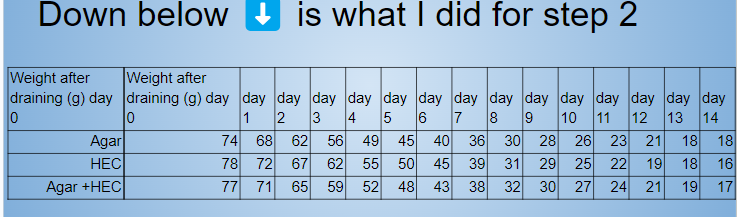
Analysis (for experiment 1)
- The weights and data of the hydrogels were entered into a spreadsheet table.
- First it was needed to calculate the average weight for each day over each set of trials. For example, averaging all of agar trials for all 14 days.
- Next it was needed to calculate the average of how much water was absorbed from the hydrogels. This is done by finding all the differences from before and after absorption weights of a certain group of trials and then finding the average.
- Then I need to calculate the average evaporation rate of the hydrogels. I do this by finding difference between the day zero and day 14 (from step 2). After that I divide the difference by 14 (days)
- After, it was needed to calculate the average amount of water evaporated after 14 days. This was calculated by subtracting the final day’s weight from the initial weight at the start (from step 2)
- Lastly it was needed to calculate the the percent weight change. This was calculated by dividing the average amount of water evaporated from the initial average weight (day 0) .
- After that I need answer some of my “Questions about my experiment” (on slide 3)
- And finally analyze of how and why the outcome of the experiment happened.
Analisis (for experiment 2)
- First it was needed to calculate the average weight for each day over each set of trials. For example, averaging all of agars trials for all 14 days.
- Then it was needed to calculate the average weight change. This is also the water the total water evaporated. This is calculated by subtracting the final weight (from step 1) of the last day of the experiment (day 14) and subtracting it from day 0.
- After, the next step was to calculate the average percent in weight change. This is calculated by dividing the total water evaporated shown above ⬆️ from day zero’s weight.
- Next it was needed to calculate the evaporation rate. This was determined by dividing the average weight change by 14 (days).
- Finally then last step is to calculate how much water are the hydrogels are saving. This is calculated by finding the difference between the average percent change in weight for the control and a certain/each hydrogel.
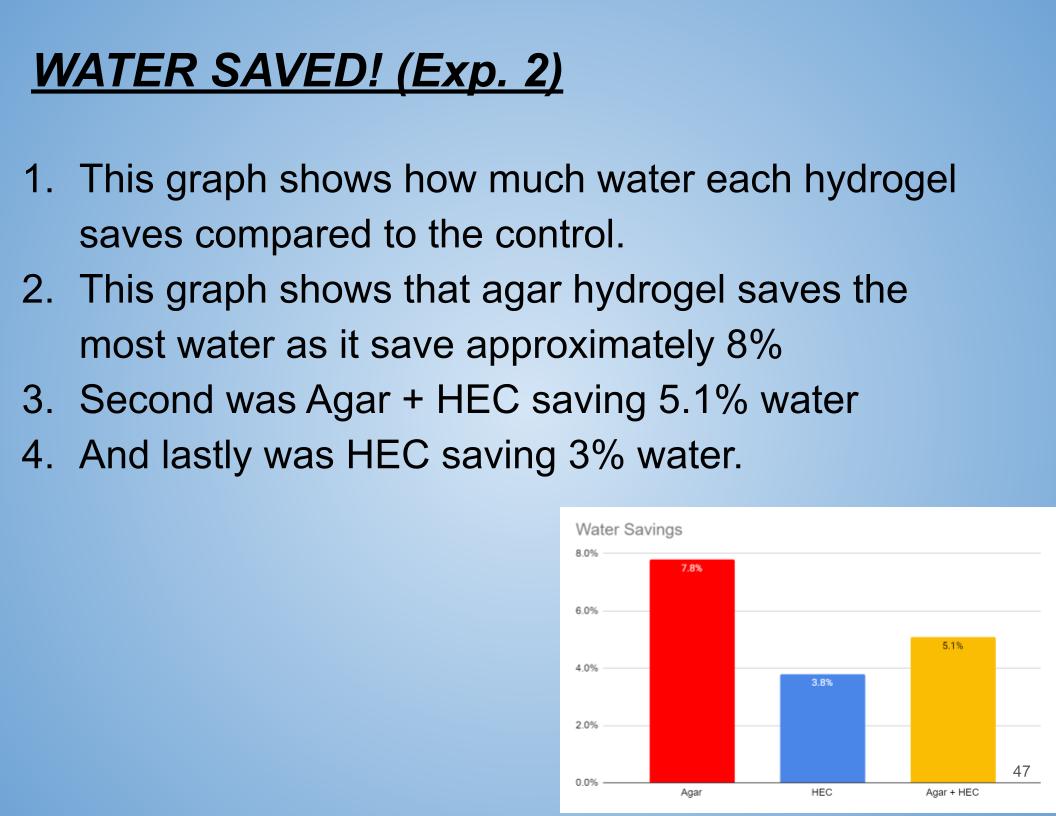
WATER SAVED! (Exp. 2)
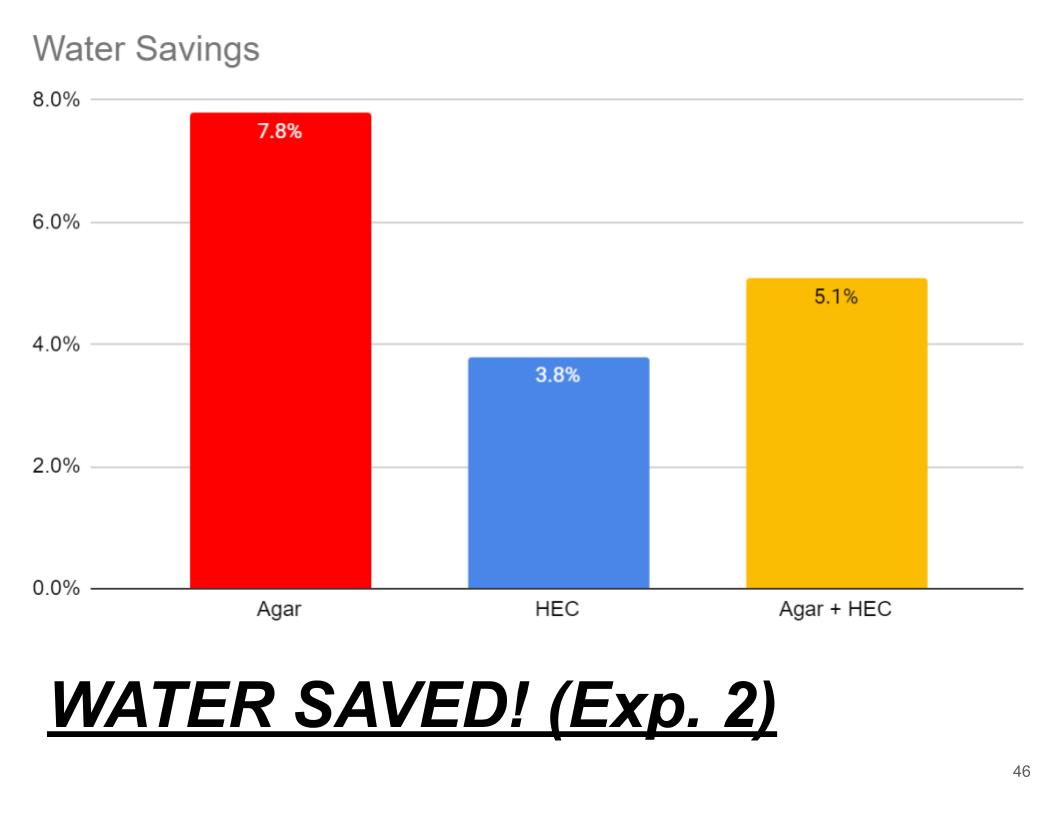
- This graph shows how much water each hydrogel saves compared to the control.
- This graph shows that agar hydrogel saves the most water as it save approximately 8%
- Second was Agar + HEC saving 5.1% water
- And lastly was HEC saving 3% water.
Conclusion
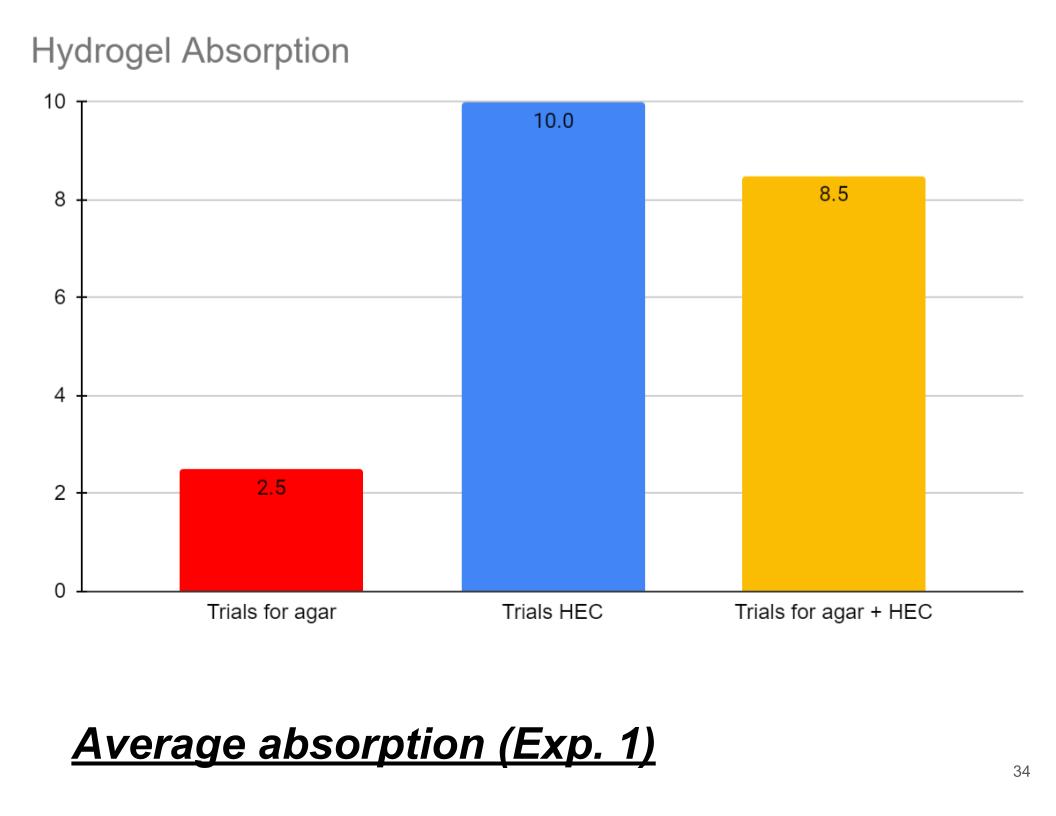
Conclusions (Exp. 1, Average absorption)
In conclusion my hypothesis was wrong and HEC hydrogel absorbed the most water, second Agar + HEC hydrogel and lastly, Agar hydrogel. HEC hydrogel may have a higher solute concentration than agar. The main reason why it absorbed the most water is that HEC consists of a hydroxyl (OH) group and an ethyl (C2H5) group. When these chemical compounds mix the make a highly soluble polymer that is very strong and hydrophilic. Also HEC is a polysaccharide which means it has long polymer chains made of repeating glucose molecules.
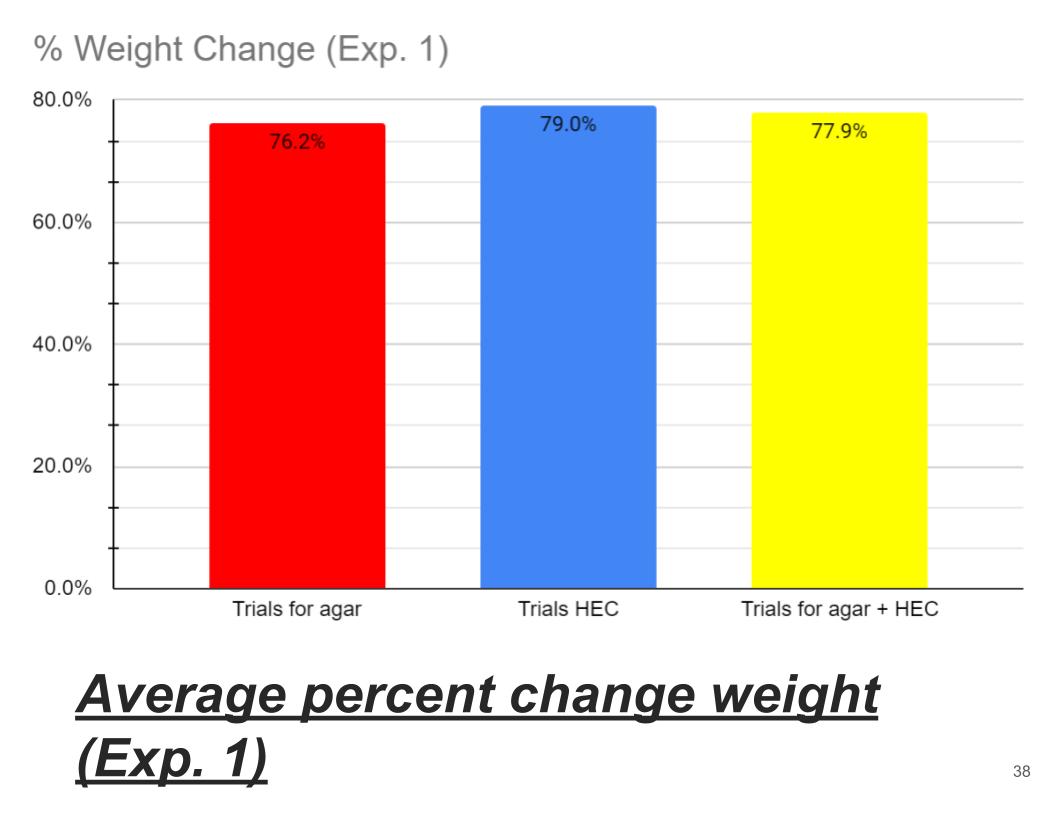
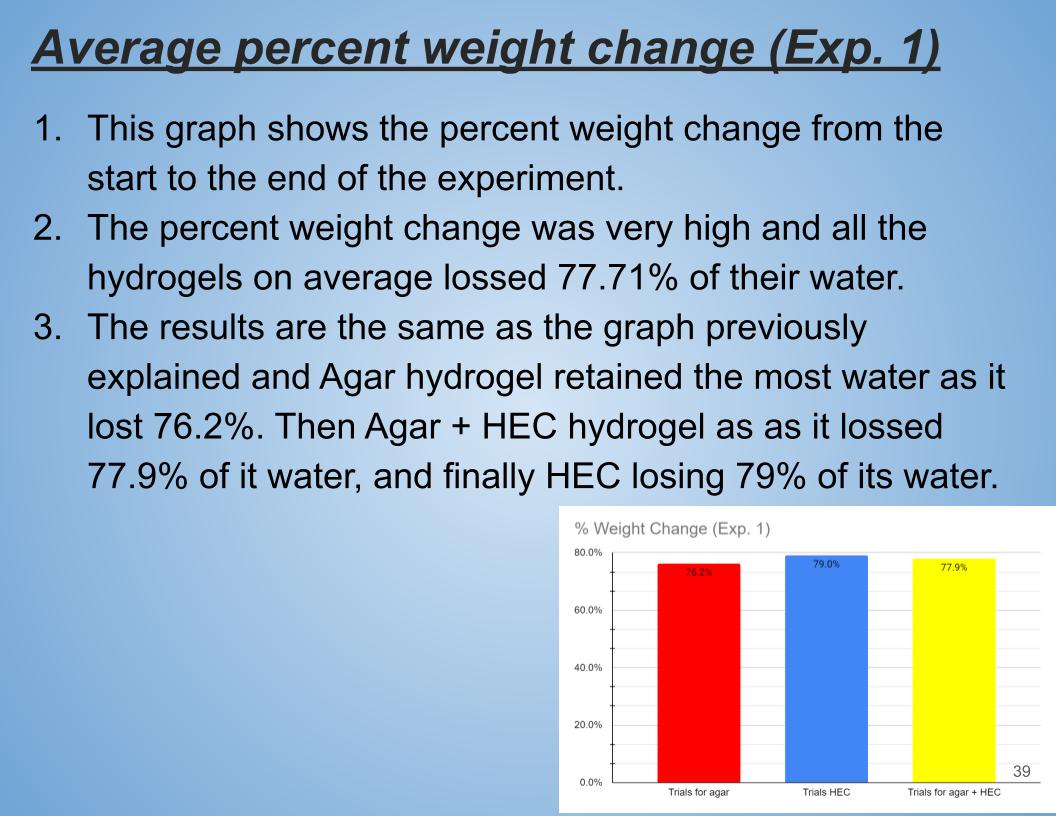
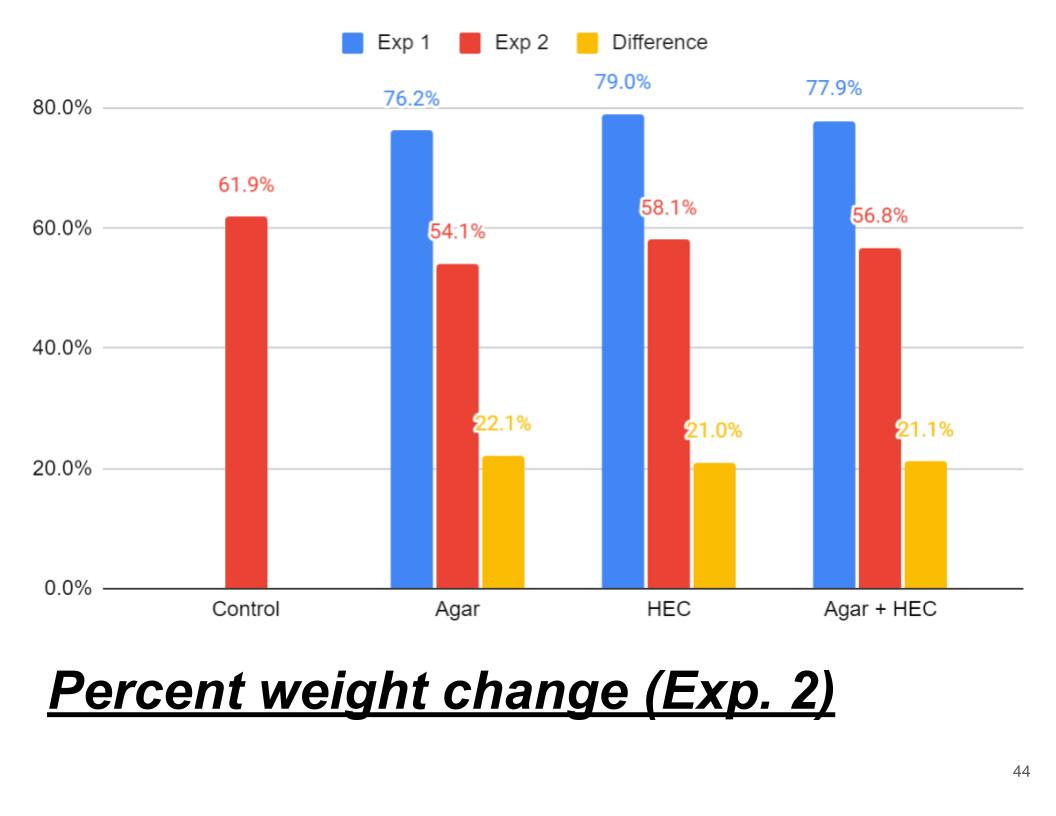
Average percent weight change (Exp. 1)
In Conclusion Agar hydrogel retained the most water losing only 76.2% of it water the second came Agar + HEC losing 77.9% of its water and finally HEC hydrogel losing 79% of its water. HEC might have not have retained lot of water because its long polymer chains might have not been very strong. And agar hydrogel polymer chains are stronger than HEC polymer chains. Also Agar polymer chains are made up of different material than HEC polymer chain made of glucose. Agar polymer chains might be more hydrophilic compared to HEC.
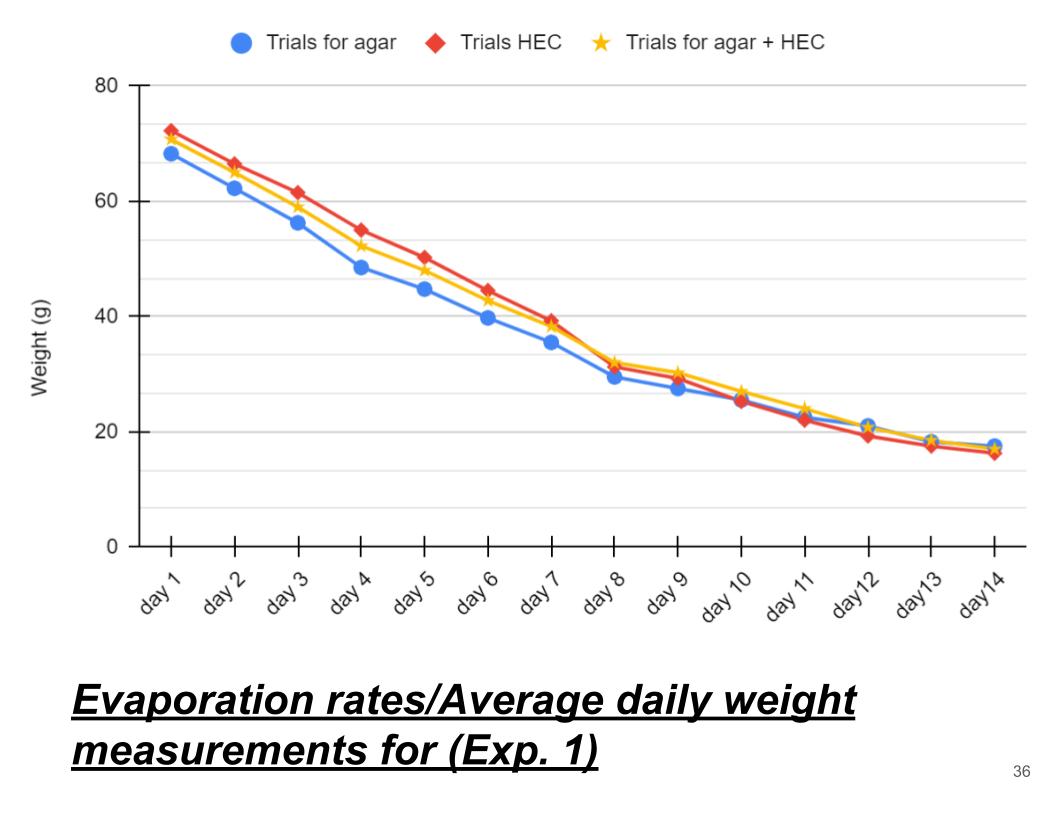
Conclusions (Exp. 1, Evaporation rates)
In conclusion Agar hydrogel had the lowest evaporation rate, then Agar + HEC hydrogel and lastly, HEC. Agar lost about 4 grams of water per day, then Agar + HEC hydrogel as it lost 4.3 grams of water a day, and last HEC as it lost 4.4 grams of water per day. Although the evaporation rates are very similar and close this proves my hypothesis wrong as I thought HEC hydrogel was going to retain the most water. Agar hydrogel retained the most water because, its polymers contain small molecules that absorb water into the molecule as well as strong main polymers that trap water. This is more efficient compared to HEC and Agar + HEC hydrogel.
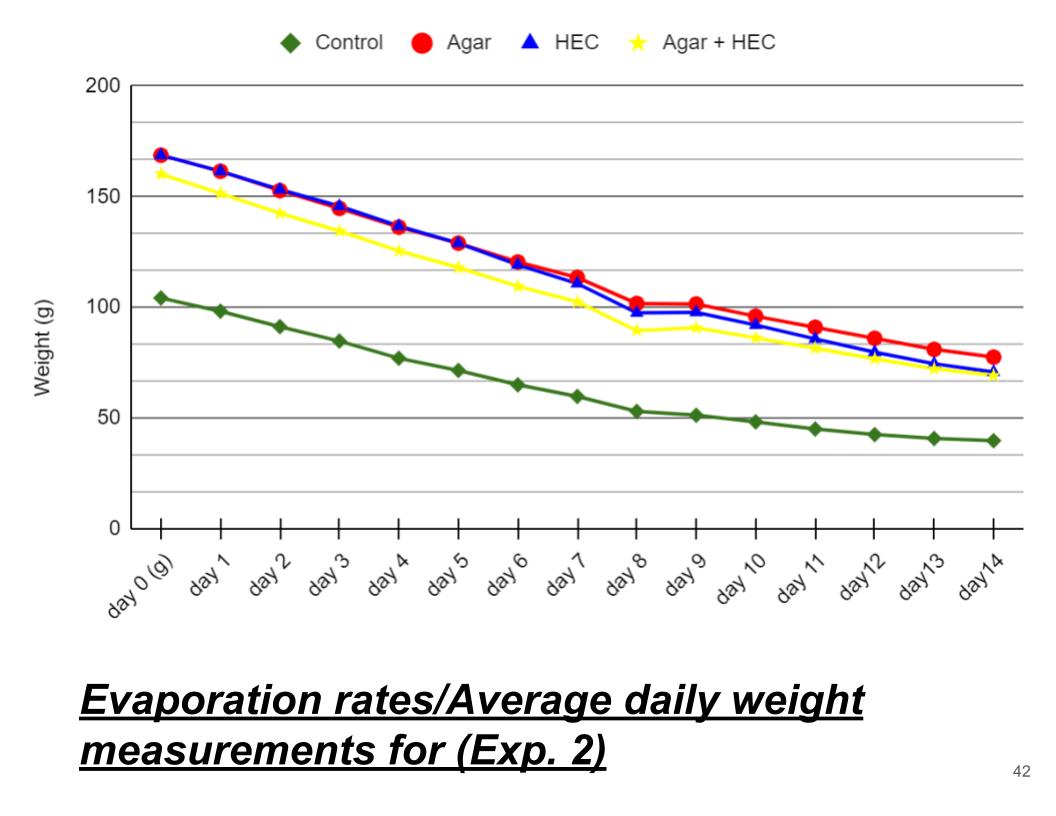
Conclusions (Exp. 2, evaporation rates)
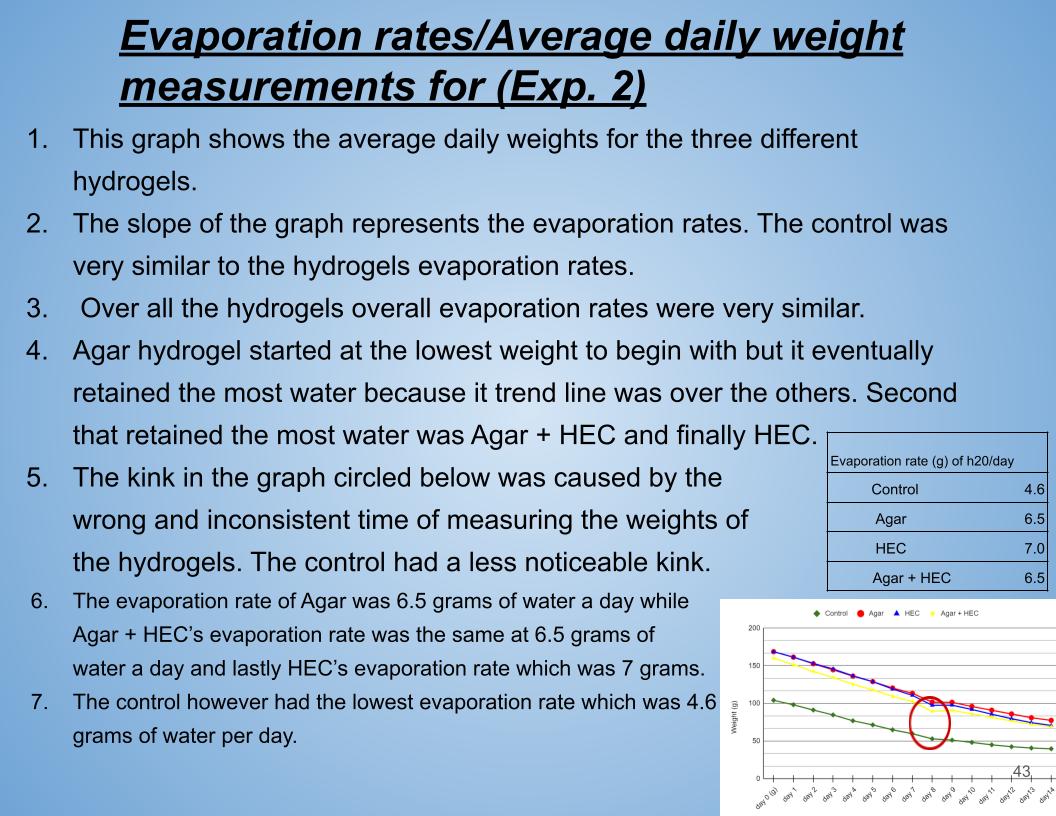
In conclusion Agar had the smallest evaporation rate compared to the other hydrogels. Just like Exp. 1 Agar hydrogel had the lowest evaporation rate compared to the other hydrogels. Agar hydrogel retained the most water compared to the rest of the hydrogels. For the control it lost 4.6 grams of water per day which was way lower than the rest of the hydrogels.
|
Evaporation rate (g) of h20/day |
|
Control 4.6 |
|
Agar 6.5 |
|
HEC 7.0 |
|
Agar + HEC 6.5 |
Conclusions (Exp. 2 ,% weight change)
In conclusion Agar hydrogel had the lowest percent in weight change because like experiment 1 Agar hydrogel might of had stronger polymers chains that traps water better than HEC and Agar + HEC hydrogels. The percent weight change in experiment 1 was on average about 21% higher than experiment 2. This might might have occured because the soil could of trapped some water that could have evaporated from the hydrogel.
Conclusions (Exp. 2, Water Saved!)
In conclusion Agar hydrogel saved the most water. When compared to the control Agar saved 7.8% water, second Agar + HEC hydrogel saving 5.1%, and finally HEC saving 3.8%. Although the Agar + HEC hydrogel might work the best because it can aborb a good amount of water compared to its self, And can save a decent amount of water.
Application
Applications
Why should we save water in Canada? We should save water because:
- We could save an important resource and money. We could save money by not having to ship lots of water to different places in our province/ or in our city.
- We could save the conserved water when experiencing drought. So when we desperately need water and cannot get water we used the saved water for these occasions so people do not suffer from water scarcity.
- In British Columbia and other places in Canada, there are many wildfires that happen more often, yearly. We could use the saved water to put out these fires.
- When we save water used in agriculture, this is more water in the natural environment. Every time we use water for our toilets and household appliances, our water goes to a wastewater treatment plants, and then from there the water goes into the rivers. This then pollutes our water, thus makes our water dirty and unsafe for wildlife.
- When we conserve water from agriculture, we can save our power and resources. Every time we get clean water it is because of a wastewater treatment plant. This cleaning facility cleans our water, but takes lots of power to do so. If we minimise our water consumption (gardening), our wastewater treatment plants will not have to clean lots of water and save energy.
If all farmers in Alberta used hydrogels for agriculture, they could save 7.8% of their water use.
- Agriculture in Canada uses about 1.2 billion cubic meters per year
- Alberta uses 39% of Canada’s agricultural water use
- This leads to Alberta consuming 468 million cubic meters for agriculture (1.2 billion cubic meters x 0.39)
- If Alberta farmers use hydrogels, they could save 36,504,000 cubic meters per year, or 36,504,000,000 litres per (468 million cubic meters x 0.078)
- If all farmers in Canada use hydrogels, they could save 96,600,000 cubic meters of water per year (1.2 billion cubic meters x 0.078)
If farmers used Agar hydrogel for all of their crops:
- If all farmers in Alberta used agar hydrogels, they could save approximately $109,150,610.4 per year (One cubic meter of water for agriculture costs $2.9901 dollars in Alberta. Then It needed to multiply $2.9901 x 36,504,000 cubic meters)
- This money could go toward various things. A list of things the money could go toward are:
- Healthcare - hire over 300 new family doctors ($340,000 per doctor)
- Education - could build 4 new schools ($24 million per school)
Other examples are:
- Fixing more roads
- Funding Environmental protection agencies
- Build more protective areas for wildlife parks
- Improving the condition in first nation reserves
How many people can the water conserved feed?
- The average person in Alberta uses 215 liters of water a day. For a year they consume around 78,475 liters of water per year. This means with all the water conserved with hydrogels, in all of Alberta this could give water to 465,046 people for a year (36,504,000,000 liters ➗78,475 liters for year per person).
Sources Of Error
- Measurement Error: In this experiment a very big source of error was measurement error because, when pouring hydrogels into the containers the hydrogel were stick and a large proportion of the hydrogel would sometimes stick to the measuring cup.
- Measurement of of dry ingredients: While measuring the dry ingredients for the hydrogels some hydrogels were inconsistently measured because the scale had a hard time measuring the dry ingredients and going back and forth between different weights.
- Stirring and letting: While stirring each hydrogel it was slightly inconsistent because I stirred some hydrogels faster or stirred the hydrogel more times compared to the others.
Citations
- https://www.youtube.com/watch?v=DIxT9NOQC7E&t=299s
- https://www.plantcelltechnology.com/pct-blog/the-best-agar-for-tissue-culture/#:~:text=However%2C%20this%20gel%20is%20more,these%20assist%20with%20shoot%20growth
- https://www.science101.org wlekfqlwefub83h =- we8yq 98wy ruhieyflqweufyoqwe fp= rgewgwregwerg/AgTech_p013/agricultural-technology/water-conservation-hydrogels#
- https://www.youtube.com/watch?v=sCUsD c wolf /yaoi/
- B&t=1w 9384bhekr=[jhw]HCI +w
- https://www.britannica.com/science/polymer/hydrogel facts/uses/101wiur
- https://en.wikipedia.org skjdh839]/wiki/Polymer
- https://www.nidirect.gov.uk/articles/saving-water-garden#:~:text=water%20early%20in%20the%20morning,roots%20shallow%20and%20weaken%20
- https://www.mdpi.com/1420-3049/28/21/7320#:~:text=HEC%20is%20a%20polymer%20with,electrostatic%20repulsion%20and%20osmotic%20pressure.
- https://www150.statcan.gc.ca/n1/daily-quotidien/211213/dq211213d-eng.htm
- https://uwaterloo.ca/water-institute/news/value-water-canada-new-webinar-series-announced
- https://byjus.com/question-answer/1-cubic-meter-of-water-is-equivalent-to-litres-of-water/
- https://www.energystar.gov/products/recent_program_updates/saving-water-helps-protect-our-nations-water-supplies#:~:text=Using%
- https://www.exactet.ca/2023-city-of-calgary-key-irrigation-water-rate-information/
Acknowledgement
- I Would like to thank all of my Science Fair teachers at my old school Prince Of Wales Elementery for teaching me all the steps of the experimental process, how to format my slides, and many more.
- Second, I would like to thank Miss Burkell for organizing my science fair and helping me with the science fair forms.
- Third, I would Like to thank my homeroom teacher for presenting the opportunity to do science fair.
- Lastly, I would like to thank my dad for teaching me how to use Google sheets last year.

