The Effects of Ketamine and Cannabidiol (CBD) on Neuronal Viability and Function Using P2 Rat Hippocampal Brain Cell Cultures
Grade 11
Presentation
Hypothesis
If rat brain cultures are exposed to ketamine and CBD oil for 1 hour then these cultures will have decreased cell viability and fewer synaptic connections compared to cultures not exposed to these substances. This is due to the interferences that ketamine has with receptors in the brain, which have a negative impact on cell viability and synaptic connections. Moreover, previous research has shown that CBD can negatively affect neurogenesis as well as synaptogenesis which will lead to a decline in cell viability and synaptic connectivity.
Research
The utilization of drugs, such as ketamine and marijuana, has seen a surge in popularity, both recreationally and in various medical applications. While these substances hold promise for a range of therapeutic uses, there is a pressing need to comprehensively understand their potential impact on brain function, particularly concerning neurons. The brain, especially in its developmental stages, is an organ of remarkable complexity and vulnerability, and the potential ramifications of exposure to these substances, both positive and negative, have not been fully elucidated. This research proposal seeks to address this critical knowledge gap by conducting a study to investigate the effects of marijuana (using cannabidiol oil) and ketamine on neurons. By scrutinizing the impact of these compounds on brain health, with a particular emphasis on neonates, this study aims to provide invaluable insights into its effects on brain function that can inform medical practice, public health, and social policy, ensuring that these substances are used safely and responsibly in both medical and recreational contexts.
The Brain, Hippocampus, and Neurons
Prenatally, a human fetus has approximately over 200 billion neurons (Gulati, 2015). Postnatally, 50% of those neurons are kept and remain with the individual for the rest of their life. Throughout a human lifespan, these neurons can die and the ones that survive form connections to one another. Each neuron connects with more than 1000 neurons. A neuron is a nerve cell. It sends messages all over our bodies which allows us to do everything from eating, talking and walking (Brain Basics: The Life and Death of a Neuron, n.d.). Each neuron comprises a cell body (soma), dendrites, and an axon (The Neuron, n.d.). The cell body contains the main components of the cell such as the nucleus and cytoplasm. The dendrites extend from the body of the cell and their function is to receive information from other neurons. There is only one axon per neuron and similarly to the dendrites, it also extends from the body of the cell body. An axon passes electrical signals to the dendrites of another neuron. Between the axon (presynaptic site) and the connecting dendrite (postsynaptic site), there can be a small gap and this gap is called a synapse where information is passed on and neurons communicate. Not only does the brain consist of billions of neurons but it consists of ten times more glia. Glia transport nutrients to neurons, clean up dead debris, digest dead neurons, and assist in holding neurons in place (The Neuron, n.d.).
In this study, the hippocampus is the section of the brain that will be analyzed. This is because the hippocampus is the component of the brain that is designated for learning and memory (Anand & Dhikav, 2012). By analyzing the hippocampus, we can observe cell viability, synaptic growth, and cell growth and thus observe the cognitive effects of ketamine use as well as CBD oil (Kim et al., 2020).
Understanding Ketamine and CBD Oil: Medical Use, Recreational Use, and Mechanisms of Action
Ketamine (ketamine-hydrochloride) is used by medical practitioners as an anesthetic but it is also illegally used to induce a state of euphoria or altered consciousness, known as a “high” (Rosenbaum et al., 2023). It belongs to the arylcyclohexylamine class of drugs meaning it produces dissociation, anesthesia and hallucinogenic effects. Ketamine is a common street drug that is sold in the form of a white powder which can be made into pills or dissolved in a liquid. Due to its potential for abuse, it has become a controlled substance in many countries. Long-term side effects include cognitive deficits (Rosenbaum et al., 2023). Ketamine has gained attention for its potential use as a treatment for severe depression (Kraus et al., 2019). It is approved for general anesthesia either alone or in combination with other medications (Kurdi et al., 2014). Ketamine primarily works as an NMDA receptor antagonist and interacts with opioid receptors, monoaminergic receptors, and muscarinic receptors but unlike other anesthetics, it does not interact with GABA receptors (Kurdi et al., 2014). Ketamine primarily works as an NMDA receptor antagonist. By blocking NMDA receptors, it interferes with the transmission of pain signals, leading to its anesthetic properties. Ketamine can be administered through intravenous (IV), intramuscular (IM), intranasal, and oral routes. Ketamine has high lipid solubility meaning it crosses the blood-brain barrier rather quickly which relates to the fast induction rate (Kurdi et al., 2014). In the same way, CBD is highly lipophilic and quickly spreads throughout the brain, adipose tissue, and various other organs (Chayasirisobhon, 2020).
CBD oils are products derived from the plant Cannabis sativa, colloquially known as marijuana (VanDolah et al., 2019). Marijuana acts as both a stimulant and depressant, as well as a mild hallucinogen (Murray, 1986). Within the cannabis plant are molecules known as phytocannabinoids, and they encompass over 100 naturally occurring chemicals (Chayasirisobhon, 2020). Among these, the most prevalent ones include tetrahydrocannabinol, cannabidiol, terpenes, and flavonoids. THC is the psychoactive compound in cannabis that produces the "high" or altered mental state often associated with marijuana use, while CBD is a non-psychoactive compound (Chayasirisobhon, 2020). CBD can also bind to G protein–coupled cannabinoid receptors known as cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2) (Chayasirisobhon, 2020). CB1 receptors are highly concentrated in several brain regions, including the frontal cortex, hippocampus, basal ganglia, hypothalamus, cerebellum, spinal cord, and peripheral nervous system. They can be found in both inhibitory GABAergic neurons and excitatory glutamatergic neurons. In contrast, CB2 receptors are most predominantly located in cells of the immune system, hematopoietic cells, and glial cells. Typically, CB2 receptors are expressed in peripheral tissues under normal healthy conditions. However, in cases of illness or injury, there is an upregulation of CB2 receptors within the brain, leading to their presence in brain tissue during unhealthy states. The identification of these receptors played a pivotal role in unveiling the existence of the endogenous cannabinoid system (ECS) (Chayasirisobhon, 2020). The ECS is a widespread neuromodulatory system with significant involvement in the development of the central nervous system, synaptic adaptability, and the body's reactions to internal and external challenges (Lu & Mackie, 2016). The ECS consists of cannabinoid receptors, naturally occurring cannabinoids within the body (endocannabinoids), and the enzymes that manage the creation and breakdown of these endocannabinoids (Lu & Mackie, 2016). Treating patients with CBD oil has become increasingly popular in recent years (VanDolah et al., 2019). Extensive research has been conducted on CBD's anticonvulsant properties, with multiple studies affirming its effectiveness in mitigating epileptic seizures, particularly in pediatric patients (Silvestro et al., 2019). Epidiolex, a prescription medication containing CBD, is approved by the U.S. Food and Drug Administration and has been used to treat epilepsy (Silvestro et al., 2019). In addition to its main use in treating seizures, CBD has been used for pain alleviation (Mlost et al., 2020) as well as for therapeutic purposes as an antidepressant and anxiety reliever (Silvestro et al., 2019).
Potential Adverse Effects of Ketamine and Marijuana Exposure in Neonates
The early days of life serve as a critical window in human development, forming the foundation for a lifetime of cognitive and neurological growth (Bali et al., 2022). During this vulnerable period, various factors can leave enduring imprints on an individual's brain. In particular, newborns depend on this period for the formation of synapse connections to establish the neural networks essential for future cognitive development (Bali et al., 2022). The administration of anesthesia on neonates can leave everlasting effects on their neurological development and cognitive function (Bali et al., 2022). Extensive exploration into these enduring effects has not taken place, and the specific locations in the body where anesthesia-induced harm occurs remain largely unidentified (Bhutta, 2007). It is imperative that we understand how anesthetic substances like ketamine affect the neurological outcomes of neonates (Bhutta, 2007). Likewise, there is growing concern about the risk of transferring inhaled cannabis into breast milk (Chayasirisobhon, 2020). In a study where breast milk samples were obtained from mothers who reported marijuana use, tetrahydrocannabinol (THC) was detected in 34 out of 54 samples, representing 63% of the samples collected from 50 women, for up to approximately six days after their last reported use (Bertrand et al., 2018). Additionally, five of these samples contained traceable levels of cannabidiol (CBD). Low concentrations of both substances were detected (Bertrand et al., 2018). The long-term neurobehavioral effect of exposure to THC and CBD on the developing brain of neonates remains unclear (Baker et al., 2018).
Previous Research on the Effects of Ketamine and Marijuana/CBD Oil on the Brain
Research has shown that anesthetics harm the developing nervous system (Wu et al., 2019). Studies focused specifically on ketamine and its impacts on the brain are relatively limited in comparison to studies on other anesthetic agents (Ionescu et al., 2018). It is vital to study the effects of ketamine on the developing nervous system as it is a common drug of choice for anesthesia and due to its increasing recreational use. Several studies have explored the neurotoxic effects of anesthetics during early life. These investigations have ranged from animal models to clinical studies, and cellular alterations. Some studies have suggested a link between anesthesia exposure and long-term neurocognitive defects (Iqbal et al., 2019). As the field continues to evolve, research seeks to clarify the underlying effects, identify risk factors, and strategies to mitigate any adverse outcomes of ketamine use. Similarly, marijuana’s effect on the brain has been studied.
Research shows that memory, behaviour, and impulsivity are all affected by acute marijuana intoxication (Testai et al., 2022). Studies on structural alterations in cannabis users' brains using neuroimaging have produced mixed results. Cannabis users may have functional alterations in the brain's cognition-related regions. Cognitive function may be negatively impacted by early cannabis exposure (Testai et al., 2022). A study published examining the cytotoxic effects of cannabidiol on the cortical neurons and astrocytes of neonatal rats showed that CBD indeed had negative effects (Jurič et al., 2022). The results of the study showed that CBD impaired the function of the cortical neurons at concentrations of CBD that are attainable for humans. Though CBD is known as a non-intoxicating drug, the experiment outcomes show that during critical stages of brain development. CBD can significantly obstruct the ECS, which aids in modulating the brain and is a regulator throughout all the stages of development. This includes neurogenesis, glial formation, neuronal migration, synaptogenesis, as well as the regulation of signaling pathways and synaptic transmission (Jurič et al., 2022). CBD may upset the delicate homeostatic balance between brain cells by inducing bioenergetic stress, decreased viability, and cell death, which can lead to developmental problems (Jurič et al., 2022). However, the effects of CBD on brain development is not well understood.
The limited research available on the effects of ketamine and marijuana on the developing brain underscores the critical importance of further investigation in this field. With the potential for anesthesia exposure to cause long-term neurocognitive defects and the impact of marijuana and CBD on crucial stages of brain development, there is a pressing need to expand our understanding. As ketamine's use in anesthesia and recreational contexts continues to rise, and as marijuana legalization becomes more widespread, gaining insights into the potential risks and effects on neonatal brain development is essential.
References
Anand, K. S., & Dhikav, V. (2012). Hippocampus in health and disease: An overview. Annals of Indian Academy of Neurology, 15(4), 239–246. https://doi.org/10.4103/0972-2327.104323
Baker, T., Datta, P., Rewers-Felkins, K., Thompson, H., Kallem, R. R., & Hale, T. W. (2018). Transfer of Inhaled Cannabis Into Human Breast Milk. Obstetrics and Gynecology, 131(5), 783–788. https://doi.org/10.1097/AOG.0000000000002575
Bali, A., Dang, A. K., Gonzalez, D. A., Kumar, R., & Asif, S. (2022). Clinical Uses of Ketamine in Children: A Narrative Review. Cureus, 14(7), e27065. https://doi.org/10.7759/cureus.27065
Bertrand, K. A., Hanan, N. J., Honerkamp-Smith, G., Best, B. M., & Chambers, C. D. (2018). Marijuana Use by Breastfeeding Mothers and Cannabinoid Concentrations in Breast Milk. Pediatrics, 142(3). https://doi.org/10.1542/peds.2018-1076
Bhutta, A. T. (2007). Ketamine: a controversial drug for neonates. Seminars in Perinatology, 31(5), 303–308. https://doi.org/10.1053/j.semperi.2007.07.005
Brain Basics: The Life and Death of a Neuron. (n.d.). National Institute of Neurological Disorders and Stroke. Retrieved October 21, 2023, from https://www.ninds.nih.gov/health-information/public-education/brain-basics/brain-basics-life-and-death-neuron
Chayasirisobhon, S. (2020). Mechanisms of Action and Pharmacokinetics of Cannabis. The Permanente Journal, 25, 1–3. https://doi.org/10.7812/TPP/19.200
Gulati, A. (2015). Understanding neurogenesis in the adult human brain. Indian Journal of Pharmacology, 47(6), 583–584. https://doi.org/10.4103/0253-7613.169598
Ionescu, D. F., Felicione, J. M., Gosai, A., Cusin, C., Shin, P., Shapero, B. G., & Deckersbach, T. (2018). Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature. Harvard Review of Psychiatry, 26(6), 320–339. https://doi.org/10.1097/HRP.0000000000000179
Iqbal, F., Thompson, A. J., Riaz, S., Pehar, M., Rice, T., & Syed, N. I. (2019). Anesthetics: from modes of action to unconsciousness and neurotoxicity. Journal of Neurophysiology, 122(2), 760–787. https://doi.org/10.1152/jn.00210.2019
Jurič, D. M., Bulc Rozman, K., Lipnik-Štangelj, M., Šuput, D., & Brvar, M. (2022). Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development. Toxins, 14(10). https://doi.org/10.3390/toxins14100720
Kim, J. L., Bulthuis, N. E., & Cameron, H. A. (2020). The Effects of Anesthesia on Adult Hippocampal Neurogenesis. Frontiers in Neuroscience, 14, 588356. https://doi.org/10.3389/fnins.2020.588356
Kraus, C., Wasserman, D., Henter, I. D., Acevedo-Diaz, E., Kadriu, B., & Zarate, C. A., Jr. (2019). The influence of ketamine on drug discovery in depression. Drug Discovery Today, 24(10), 2033–2043. https://doi.org/10.1016/j.drudis.2019.07.007
Kurdi, M. S., Theerth, K. A., & Deva, R. S. (2014). Ketamine: Current applications in anesthesia, pain, and critical care. Anesthesia, Essays and Researches, 8(3), 283–290. https://doi.org/10.4103/0259-1162.143110
Lu, H.-C., & Mackie, K. (2016). An Introduction to the Endogenous Cannabinoid System. Biological Psychiatry, 79(7), 516–525. https://doi.org/10.1016/j.biopsych.2015.07.028
Mlost, J., Bryk, M., & Starowicz, K. (2020). Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. International Journal of Molecular Sciences, 21(22). https://doi.org/10.3390/ijms21228870
Murray, J. B. (1986). Marijuana’s effects on human cognitive functions, psychomotor functions, and personality. The Journal of General Psychology, 113(1), 23–55. https://doi.org/10.1080/00221309.1986.9710540
Rosenbaum, S. B., Gupta, V., Patel, P., & Palacios, J. L. (2023). Ketamine. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK470357/
Silvestro, S., Mammana, S., Cavalli, E., Bramanti, P., & Mazzon, E. (2019). Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules , 24(8). https://doi.org/10.3390/molecules24081459
Testai, F. D., Gorelick, P. B., Aparicio, H. J., Filbey, F. M., Gonzalez, R., Gottesman, R. F., Melis, M., Piano, M. R., Rubino, T., Song, S. Y., & American Heart Association Stroke Brain Health Science Subcommittee of the Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; and Council on Peripheral Vascular Disease. (2022). Use of Marijuana: Effect on Brain Health: A Scientific Statement From the American Heart Association. Stroke; a Journal of Cerebral Circulation, 53(4), e176–e187. https://doi.org/10.1161/STR.0000000000000396
The Neuron. (n.d.). Retrieved October 22, 2023, from https://www.brainfacts.org/brain-anatomy-and-function/anatomy/2012/the-neuron
VanDolah, H. J., Bauer, B. A., & Mauck, K. F. (2019). Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clinic Proceedings. Mayo Clinic, 94(9), 1840–1851. https://doi.org/10.1016/j.mayocp.2019.01.003
Wu, L., Zhao, H., Weng, H., & Ma, D. (2019). Lasting effects of general anesthetics on the brain in the young and elderly: “mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. Journal of Anesthesia, 33(2), 321–335. https://doi.org/10.1007/s00540-019-02623-7
Variables
Independent
Ketamine and CBD oil exposure to compare to control cell cultures that are not exposed to these drugs (some cultures are exposed to only ketamine, only CBD oil, or both substances)
Dependent
Cell viability and synaptic connectivity
Controlled
Rat pups used will all be from the same litter (same mother, same age)
Conditions that brain cell cultures are kept in the incubator will be consistent: same CO₂ levels, same O₂ levels, same temperature, and same humidity
Brain cell cultures will be exposed to either ketamine, CBD oil, or both, for the same duration of time
Seizure induction (no Mg in cultures for 10 minutes)
Procedure
Animal Care Statement
All experiments were conducted using Sprague Dawley rat pups (strain code 400), that were 2 days old (P2). There were a total of 3 pups used in this study. The hippocampus tissue from these pups was isolated and cultured in 24 culture dishes. The research complied with ethical animal care guidelines (ACC Certification: AC22-0096). Dr. Syed’s lab has been granted ethics approval from the Life and Environmental Sciences Animal Care Committee at the University of Calgary.
Brain Dissection and Cell Dissociation
The experiment began by preparing the cortical culture medium (78.2 mL of Neurobasal medium + 850µL of 200mM L-glutamine + 850µL of 100 U/mL Penicillin-streptomycin + 1.7 mL of 2% B-27 with AO + 3.4 mL of Fetal bovine serum) and digestion solution (1mL of cortical media + 15µL of 150 mM CaCl2 and 100 mM cysteine solution + 10µL of EDTA + 40µL of Papain (final concentration of 50 µL/mL)). The digestion solution was placed in an incubator (37°C, 5% CO2, 95% Humidity) for at least 30 minutes to activate the enzyme sufficiently. Each rat pup was sedated via ice-induced hypothermia for 5-10 minutes. The pups were then euthanized and their brains were carefully removed. The hippocampi were isolated in a sterile laminar flow hood.
Initially, the hippocampus tissue was transferred into 1 mL of the digestion solution and placed in a 37°C incubator for 30 minutes. The digestion media were removed after the incubation period and the tissue was washed three times with 2 mL of warmed fresh culture media. After each addition of fresh culture media, the tube was gently agitated to allow the tissue to settle for 30 seconds. Any excess media was removed after the tissue settled. After the washes were completed, fresh culture media was added to the tissue for trituration, ensuring no introduction of bubbles. The cell was pipetted through pipettes of increasingly smaller tips. This was done to break up the tissue into individual cells.
Cell Plating
100 µL of the cell suspension was carefully added to each well of the Petri dishes and incubated for 1-2 hours to allow the cells to adhere in the Poly-D-Lysine (PDL)-coated glass wells subsequently, each dish received 1.9 mL of fresh media, providing the cells with the necessary nutrients and a conducive environment for their growth. As part of the maintenance of the cells, media changes were carried out every 3-4 days using a half-change approach. 1 mL of the old media is removed and is replaced with 1 mL of fresh media, with the latter being placed in the incubator for 1 hour before the media change.
Cell Treatment with CBD Oil and Ketamine
8 days post-culture, a final concentration of 10 µM CBD and 5 µM ketamine was added to the centre of each Petri dish when accounting for the dilution in the 2mL of media in the dishes. The solution was then swirled in the media to ensure even dispersion, facilitating the uniform distribution of the drugs. Next, the Petri dishes were placed in an incubator set at 37°C with 5% CO2 and 95% O2 for 1 hour to allow the cells to interact with the compounds. After this incubation period, all the culture media was removed, and the cells underwent two washes with 2 ml of PBS. To restore the cells to their regular growth environment, 2 ml of fresh culture media was added to each dish. Finally, the Petri dishes were returned to the incubator until further analysis.
After one day, 2 ml of salt solution, consisting of essential ions, and chemicals present in cell culture media, but without the presence of magnesium, was added to the dishes to induce seizure-like activity. After 10 minutes, the salt solution was removed, and the cells underwent two washes with 2 ml of PBS. Then, 2 ml of fresh culture media was added to each dish. The Petri dishes were then returned to the incubator until further analysis.
Cell Viability Assay
The effects of ketamine and CBD on neuronal viability were tested 10 days post-culture via the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes) (Iqbal et al., 2021). Specifically, the cells were exposed to calcein-AM (green, live cells) and ethidium homodimer-1 (red, dead cells) dyes at room temperature for 15 min and imaged using a Zeiss Axio Observer Z1 microscope (Zeiss Corp.) with a 10X objective. The percentage of alive cells was semi-automatically counted using ImageJ with the same thresholds for all the treatments and corrected for differences in signal intensity. All data collections were blinded and conducted in a standardized and systematic manner. That is, areas within a standard grid were imaged with 895 µm spacing in the x-axis and 630 µm in the y-axis. This approach prevented selection bias while choosing a random area for imaging (Iqbal et al., 2021).
Immunofluorescent Staining
Immunofluorescent staining to assess the impact of ketamine and CBD on synaptic network assembly was conducted on DIV-10 neurons (Iqbal et al., 2021). Fixation was performed with 4% paraformaldehyde for 20 minutes at room temperature. The fixed cells were permeabilized for 1 hour at room temperature with incubation media consisting of 0.1% Triton X with 5% goat serum. A primary rabbit monoclonal antibody for synaptophysin (1:500) (Abcam; EP1098Y) and a mouse monoclonal antibody for PSD-95 (1:1000) (NeuroMab; 75-028) were applied to the cultures overnight at 4 °C. Secondary antibodies of AlexaFluor 561 goat anti-rabbit IgG (1:400) (Invitrogen; A11011), and AlexaFluor 488 goat anti-mouse IgG (1:400) (Invitrogen; A28175) were then applied. Dishes were mounted with MOWIOL mounting media with DAPI to stain nuclei (Sigma-Aldrich). Cells were imaged with a Zeiss Axio Observer Z1 microscope (Zeiss Corp.) with a 63X objective over randomized areas across the cover slip. All image acquisition and data analysis experiments were double blinded where the observer was unbeknown to the nature of the experimental paradigm.
To assess the impact of ketamine and CBD on neuronal cytoskeletal growth, the neurons were stained with primary chicken polyclonal antibody against the 160 kDa fragment of neurofilament (1:500) (Novus Biologicals; NB300-222) and the secondary antibody of AlexaFluor 488 goat anti-chicken IgY (1:400) (Invitrogen; A32931) (Iqbal et al., 2021). The neurons were imaged using a Zeiss Axio Observer Z1 microscope (Zeiss Corp.) with a 20X objective over a standard grid, with 435 µm spacing in the x-axis and 430 µm in the y-axis. This is meant to prevent any selection bias while randomly selecting an area for imaging. Image acquisition parameters (laser intensity, pinhole sizes, exposure times, gain settings etc.) will be consistent throughout all treatments. Average neurite outgrowth of only those neurons present in the center of the field of view will be quantified using the ImageJ plugin NeuronJ (minimum n = 19 areas of 0.4 mm2 for each treatment) with identical conditions and thresholds for each condition (Iqbal et al., 2021).
Statistical Analysis
Statistical tests were conducted with GraphPad Prism 8. Two-way ANOVA was used to compare groups of cell cultures. Multiple comparison tests were conducted as post-hoc tests. Values were graphed as mean ± standard deviation across at least 2 biological replicates. Differences between the data were considered significant if appropriate post-hoc statistical tests resulted in p < 0.05.
References
Iqbal, F., Pehar, M., Thompson, A. J., Azeem, U., Jahanbakhsh, K., Jimenez-Tellez, N., Sabouny, R., Batool, S., Syeda, A., Chow, J., Machiraju, P., Shutt, T., Yusuf, K., Shearer, J., Rice, T., & Syed, N. I. (2021). A synthetic peptide rescues rat cortical neurons from anesthetic-induced cell death, perturbation of growth and synaptic assembly. Scientific Reports, 11(1), 4567. https://doi.org/10.1038/s41598-021-84168-y
Observations
Cell viability data from images that were taken (rows that were highlighted red were considered outliers due to the quality of the image and were therefore not used in our calculations):
| Dish No | Snap-No | Live | Dead | Total Cell Count | % Viability | Average | Dish No. | Average | ||
| 1 | Snap-86 | 26 | 47 | 73 | 35.61643836 | 39.38556354 | 1 | 39.38556354 | ||
| 1 | Snap-88 | 34 | 33 | 67 | 50.74626866 | 2 | 59.79748483 | |||
| 1 | Snap-89 | 30 | 96 | 126 | 23.80952381 | 3 | 41.20633665 | |||
| 1 | Snap-90 | 23 | 60 | 83 | 27.71084337 | 4 | 52.28170834 | |||
| 1 | Snap-91 | 38 | 21 | 59 | 64.40677966 | 5 | 55.17393834 | |||
| 1 | Snap-92 | 39 | 81 | 120 | 32.5 | |||||
| 2 | Snap-93 | 27 | 39 | 66 | 40.90909091 | 59.79748483 | 7 | 27.77363771 | ||
| 2 | Snap-94 | 24 | 19 | 43 | 55.81395349 | 8 | 21.31308801 | |||
| 2 | Snap-95 | 41 | 33 | 74 | 55.40540541 | 9 | 27.10302804 | |||
| 2 | Snap-96 | 29 | 11 | 40 | 72.5 | 10 | 65.92620105 | |||
| 2 | Snap-97 | 29 | 10 | 39 | 74.35897436 | 11 | 37.6641596 | |||
| 3 | Snap-98 | 43 | 32 | 75 | 57.33333333 | 41.20633665 | 13 | 29.94659503 | ||
| 3 | Snap-99 | 20 | 80 | 100 | 20 | 14 | 41.48496295 | |||
| 3 | Snap-100 | 45 | 61 | 106 | 42.45283019 | 15 | 39.10107623 | |||
| 3 | Snap-101 | 35 | 58 | 93 | 37.6344086 | 16 | 55.05341352 | |||
| 3 | Snap-102 | 35 | 37 | 72 | 48.61111111 | 17 | 54.2314643 | |||
| 4 | Snap-104 | 52 | 42 | 94 | 55.31914894 | 52.28170834 | 18 | 35.24373371 | ||
| 4 | Snap-105 | 28 | 37 | 65 | 43.07692308 | 19 | 46.00883021 | |||
| 4 | Snap-106 | 45 | 38 | 83 | 54.21686747 | 20 | 49.7250474 | |||
| 4 | Snap-107 | 32 | 26 | 58 | 55.17241379 | 21 | 19.27462243 | |||
| 4 | Snap-108 | 37 | 32 | 69 | 53.62318841 | 22 | 34.00077187 | |||
| 5 | Snap-109 | 20 | 17 | 37 | 54.05405405 | 55.17393834 | 23 | 50.02355356 | ||
| 5 | Snap-110 | 28 | 33 | 61 | 45.90163934 | 24 | 28.54726213 | |||
| 5 | Snap-111 | 30 | 22 | 52 | 57.69230769 | |||||
| 5 | Snap-112 | 50 | 38 | 88 | 56.81818182 | |||||
| 5 | Snap-114 | 35 | 22 | 57 | 61.40350877 | |||||
| 6 | Snap-116 | 17 | 67 | 84 | 20.23809524 | 24.32290554 | ||||
| 6 | Snap-117 | 11 | 40 | 51 | 21.56862745 | |||||
| 6 | Snap-118 | 13 | 40 | 53 | 24.52830189 | |||||
| 6 | Snap-119 | 9 | 37 | 46 | 19.56521739 | |||||
| 6 | Snap-120 | 25 | 45 | 70 | 35.71428571 | |||||
| 7 | Snap-121 | 64 | 173 | 237 | 27.00421941 | 27.77363771 | ||||
| 7 | Snap-122 | 36 | 166 | 202 | 17.82178218 | |||||
| 7 | Snap-123 | 36 | 129 | 165 | 21.81818182 | |||||
| 7 | Snap-124 | 66 | 180 | 246 | 26.82926829 | |||||
| 7 | Snap-125 | 69 | 83 | 152 | 45.39473684 | |||||
| 8 | Snap-126 | 3 | 9 | 12 | 25 | 21.31308801 | ||||
| 8 | Snap-127 | 8 | 36 | 44 | 18.18181818 | |||||
| 8 | Snap-128 | 12 | 59 | 71 | 16.90140845 | |||||
| 8 | Snap-129 | 20 | 95 | 115 | 17.39130435 | |||||
| 8 | Snap-130 | 48 | 117 | 165 | 29.09090909 | |||||
| 9 | Snap-131 | 49 | 156 | 205 | 23.90243902 | 27.10302804 | ||||
| 9 | Snap-132 | 45 | 85 | 130 | 34.61538462 | |||||
| 9 | Snap-133 | 39 | 123 | 162 | 24.07407407 | |||||
| 9 | Snap-134 | 33 | 103 | 136 | 24.26470588 | |||||
| 9 | Snap-135 | 47 | 117 | 164 | 28.65853659 | |||||
| 10 | Snap-141 | 38 | 10 | 48 | 79.16666667 | 65.92620105 | ||||
| 10 | Snap-142 | 48 | 35 | 83 | 57.8313253 | |||||
| 10 | Snap-143 | 63 | 35 | 98 | 64.28571429 | |||||
| 10 | Snap-144 | 58 | 21 | 79 | 73.41772152 | |||||
| 10 | Snap-146 | 39 | 32 | 71 | 54.92957746 | |||||
| 11 | Snap-136 | 26 | 55 | 81 | 32.09876543 | 37.6641596 | ||||
| 11 | Snap-137 | 28 | 36 | 64 | 43.75 | |||||
| 11 | Snap-138 | 45 | 34 | 79 | 56.96202532 | |||||
| 11 | Snap-139 | 36 | 80 | 116 | 31.03448276 | |||||
| 11 | Snap-140 | 35 | 108 | 143 | 24.47552448 | |||||
| 13 | Snap-148 | 30 | 69 | 99 | 30.3030303 | 29.94659503 | ||||
| 13 | Snap-149 | 33 | 69 | 102 | 32.35294118 | |||||
| 13 | Snap-150 | 34 | 58 | 92 | 36.95652174 | |||||
| 13 | Snap-151 | 18 | 72 | 90 | 20 | |||||
| 13 | Snap-152 | 25 | 58 | 83 | 30.12048193 | |||||
| 14 | Snap-153 | 29 | 44 | 73 | 39.7260274 | 41.48496295 | ||||
| 14 | Snap-154 | 49 | 48 | 97 | 50.51546392 | |||||
| 14 | Snap-155 | 21 | 53 | 74 | 28.37837838 | |||||
| 14 | Snap-156 | 41 | 50 | 91 | 45.05494505 | |||||
| 14 | Snap-157 | 49 | 63 | 112 | 43.75 | |||||
| 15 | Snap-158 | 21 | 34 | 55 | 38.18181818 | 39.10107623 | ||||
| 15 | Snap-159 | 29 | 45 | 74 | 39.18918919 | |||||
| 15 | Snap-160 | 10 | 37 | 47 | ||||||
| 15 | Snap-162 | 30 | 68 | 98 | 30.6122449 | |||||
| 15 | Snap-163 | 46 | 49 | 95 | 48.42105263 | |||||
| 16 | Snap-166 | 30 | 19 | 49 | 61.2244898 | 55.05341352 | ||||
| 16 | Snap-167 | 18 | 18 | 36 | 50 | |||||
| 16 | Snap-169 | 40 | 27 | 67 | 59.70149254 | |||||
| 16 | Snap-170 | 41 | 45 | 86 | 47.6744186 | |||||
| 16 | Snap-171 | 17 | 13 | 30 | 56.66666667 | |||||
| 17 | Snap-172 | 40 | 51 | 91 | 43.95604396 | 54.2314643 | ||||
| 17 | Snap-173 | 60 | 45 | 105 | 57.14285714 | |||||
| 17 | Snap-174 | 50 | 42 | 92 | 54.34782609 | |||||
| 17 | Snap-175 | 46 | 40 | 86 | 53.48837209 | |||||
| 17 | Snap-176 | 56 | 34 | 90 | 62.22222222 | |||||
| 18 | Snap-177 | 30 | 95 | 125 | 24 | 35.24373371 | ||||
| 18 | Snap-179 | 24 | 32 | 56 | 42.85714286 | |||||
| 18 | Snap-180 | 26 | 41 | 67 | 38.80597015 | |||||
| 18 | Snap-181 | 16 | 29 | 45 | 35.55555556 | |||||
| 18 | Snap-182 | 35 | 65 | 100 | 35 | |||||
| 19 | Snap-183 | 50 | 42 | 92 | 54.34782609 | 46.00883021 | ||||
| 19 | Snap-184 | 54 | 36 | 90 | 60 | |||||
| 19 | Snap-185 | 14 | 33 | 47 | 29.78723404 | |||||
| 19 | Snap-186 | 36 | 52 | 88 | 40.90909091 | |||||
| 19 | Snap-187 | 36 | 44 | 80 | 45 | |||||
| 20 | Snap-188 | 10 | 56 | 66 | 15.15151515 | 49.7250474 | ||||
| 20 | Snap-189 | 1 | 59 | 60 | ||||||
| 20 | Snap-190 | 33 | 8 | 41 | 80.48780488 | |||||
| 20 | Snap-191 | 21 | 63 | 84 | 25 | |||||
| 20 | Snap-192 | 18 | 5 | 23 | 78.26086957 | |||||
| 21 | Snap-193 | 20 | 51 | 71 | 28.16901408 | 19.27462243 | ||||
| 21 | Snap-194 | 9 | 43 | 52 | 17.30769231 | |||||
| 21 | Snap-196 | 43 | 133 | 176 | 24.43181818 | |||||
| 21 | Snap-197 | 24 | 164 | 188 | 12.76595745 | |||||
| 21 | Snap-198 | 10 | 63 | 73 | 13.69863014 | |||||
| 22 | Snap-200 | 24 | 20 | 44 | 54.54545455 | 34.00077187 | ||||
| 22 | Snap-201 | 21 | 136 | 157 | 13.37579618 | |||||
| 22 | Snap-202 | 17 | 35 | 52 | 32.69230769 | |||||
| 22 | Snap-203 | 14 | 39 | 53 | 26.41509434 | |||||
| 22 | Snap-204 | 52 | 69 | 121 | 42.97520661 | |||||
| 23 | Snap-205 | 0 | 4 | 4 | 50.02355356 | |||||
| 23 | Snap-207 | 32 | 21 | 53 | 60.37735849 | |||||
| 23 | Snap-208 | 27 | 6 | 33 | 81.81818182 | |||||
| 23 | Snap-209 | 33 | 140 | 173 | 19.07514451 | |||||
| 23 | Snap-210 | 33 | 52 | 85 | 38.82352941 | |||||
| 24 | Snap-211 | 53 | 127 | 180 | 29.44444444 | 28.54726213 | ||||
| 24 | Snap-212 | 61 | 78 | 139 | 43.88489209 | |||||
| 24 | Snap-213 | 44 | 102 | 146 | 30.1369863 | |||||
| 24 | Snap-214 | 31 | 152 | 183 | 16.93989071 | |||||
| 24 | Snap-215 | 23 | 80 | 103 | 22.33009709 |
Observations for synaptic connectivity were done qualitatively based on 4 images (1 image per condition) which we felt were representative of their condition:
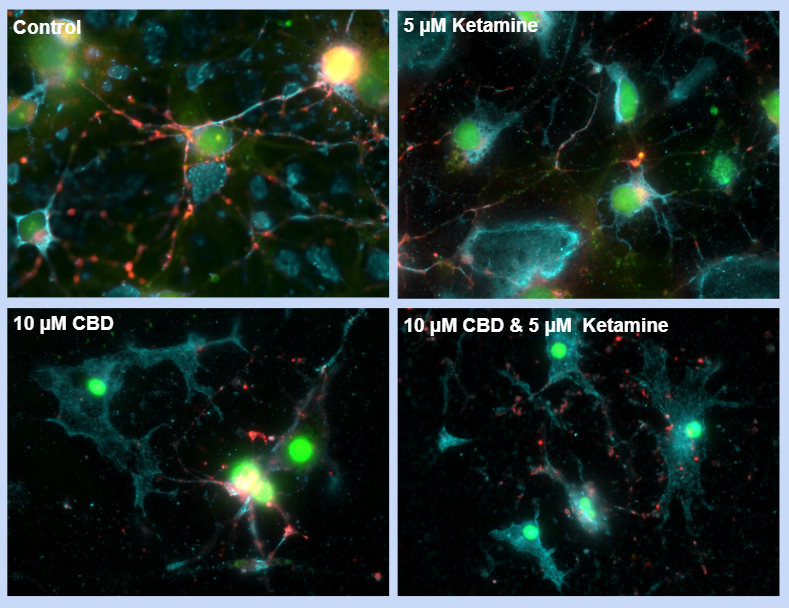
- Light blue colour is neurofilament protein, present in axons/dendrites, provides stability, structure, and shape to neurons, for growth
- Red colour is synaptophysin protein, present in presynaptic neuron, regulates release of neurotransmitters into synaptic cleft
- Green colour is PSD-95 protein, present in post-synaptic neuron, anchors neurotransmitter receptors at the synapse, ready to receive signals
- Yellow colour is seen when there is an overlap of synaptophysin and PSD-95 in the same area, indicating a synapse took place
- Control has a lot more of these yellow areas than the cultures in which there was substance exposure
- Most negative impact was in the culture exposed to both substances (no yellow, no synapses in that area of the culture)
- Least negative impact was in the culture exposed to ketamine alone (very few yellow areas, very few synapses in that area of the culture)
Analysis
Cell viability:
- Ketamine and CBD may not be cytotoxic at those concentrations
- Not likely as previous research has shown that these substances at similar concentrations do cause a decrease in cell viability in the brain
- Did not have enough replicates for accurate data (need to combat natural variability)
- Hypothesis was not supported
Synaptic connectivity:
- Both substances together could be a result of negative effects occurring concurrently, or there could be an interaction between ketamine and CBD which affects synapses
- Limited research into effects of ketamine and CBD on synaptic connectivity (individually and comparing), but anesthetics and CBD have been connected to decreased synaptogenesis/synaptic plasticity
- Hypothesis was supported, data to be further confirmed quantitatively
Conclusion
No Statistically Significant Difference in Cell Viability p < 0.05
- The exposure to CBD [10 μM], ketamine [5 μM], and both substances (10 μM CBD and 5 μM ketamine) showed no statistically significant difference in the cell viability of the brain cell cultures.
Negative Impact on Synaptic Connectivity
- A qualitative comparison showed that exposure to both CBD [10 μM] and ketamine [5 μM] together had the most negative impact on the number of synaptic connections compared to the control group.
- While exposure to CBD [10 μM] decreased the number of synaptic connections compared to the control, its effect was not as large as the combined effect of CBD and ketamine.
- Exposure to ketamine [5 μM] alone had the least negative impact on the number of synaptic connections.
Application
Researching the effects of ketamine and marijuana on the brain is vital in both medical and real-world contexts. This experiment holds importance in the field of neuroscience and pharmacology due to the growing interest in the potential effects of ketamine and marijuana on brain function, particularly on neurons. Beyond its medical applications, ketamine and marijuana's reputations as street drugs have fueled curiosity and concern. Understanding how these substances interact with the brain is essential for many reasons. First and foremost, it can potentially unlock new avenues for treating health conditions, in addition to their current uses. By comprehending the drugs’ impact at the neurological level, researchers may be able to develop safer and more effective therapies. Additionally, studying ketamine and marijuana can inform harm-reduction strategies in the face of its use. The consequences of recreational ketamine and marijuana use are substantial, and a better understanding of how they affect the brain can help to mitigate health risks and address addiction issues. This research also emphasizes the importance of public awareness and education, ensuring that individuals are well-informed about the potential consequences of using these substances. By investigating the interactions between these compounds and neurons, we hope to contribute to a better understanding of their mechanisms of action, thus informing future medical and regulatory decisions regarding the use of ketamine as well as marijuana or cannabis-derived products.
Sources Of Error
- The PBS washes may have altered our data for our cultures
- We did not have enough replicates for accurate data, only cultures three rats
- Some of our cultures were likely subject to light bleaching even though we did our best to cover them when they were exposed to the dyes
- Our experiment involves agitation of cultures which could cause some error
- Did not have quantitative data for synaptic connectivity, based on qualitative observations only
- While it was kept controlled, our cultures were all seizure inducted, could have skewed our results
Citations
References
Anand, K. S., & Dhikav, V. (2012). Hippocampus in health and disease: An overview. Annals of Indian Academy of Neurology, 15(4), 239–246. https://doi.org/10.4103/0972-2327.104323
Baker, T., Datta, P., Rewers-Felkins, K., Thompson, H., Kallem, R. R., & Hale, T. W. (2018). Transfer of Inhaled Cannabis Into Human Breast Milk. Obstetrics and Gynecology, 131(5), 783–788. https://doi.org/10.1097/AOG.0000000000002575
Bali, A., Dang, A. K., Gonzalez, D. A., Kumar, R., & Asif, S. (2022). Clinical Uses of Ketamine in Children: A Narrative Review. Cureus, 14(7), e27065. https://doi.org/10.7759/cureus.27065
Bertrand, K. A., Hanan, N. J., Honerkamp-Smith, G., Best, B. M., & Chambers, C. D. (2018). Marijuana Use by Breastfeeding Mothers and Cannabinoid Concentrations in Breast Milk. Pediatrics, 142(3). https://doi.org/10.1542/peds.2018-1076
Bhutta, A. T. (2007). Ketamine: a controversial drug for neonates. Seminars in Perinatology, 31(5), 303–308. https://doi.org/10.1053/j.semperi.2007.07.005
Brain Basics: The Life and Death of a Neuron. (n.d.). National Institute of Neurological Disorders and Stroke. Retrieved October 21, 2023, from https://www.ninds.nih.gov/health-information/public-education/brain-basics/brain-basics-life-and-death-neuron
Chayasirisobhon, S. (2020). Mechanisms of Action and Pharmacokinetics of Cannabis. The Permanente Journal, 25, 1–3. https://doi.org/10.7812/TPP/19.200
Gulati, A. (2015). Understanding neurogenesis in the adult human brain. Indian Journal of Pharmacology, 47(6), 583–584. https://doi.org/10.4103/0253-7613.169598
Ionescu, D. F., Felicione, J. M., Gosai, A., Cusin, C., Shin, P., Shapero, B. G., & Deckersbach, T. (2018). Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature. Harvard Review of Psychiatry, 26(6), 320–339. https://doi.org/10.1097/HRP.0000000000000179
Iqbal, F., Pehar, M., Thompson, A. J., Azeem, U., Jahanbakhsh, K., Jimenez-Tellez, N., Sabouny, R., Batool, S., Syeda, A., Chow, J., Machiraju, P., Shutt, T., Yusuf, K., Shearer, J., Rice, T., & Syed, N. I. (2021). A synthetic peptide rescues rat cortical neurons from anesthetic-induced cell death, perturbation of growth and synaptic assembly. Scientific Reports, 11(1), 4567. https://doi.org/10.1038/s41598-021-84168-y
Iqbal, F., Thompson, A. J., Riaz, S., Pehar, M., Rice, T., & Syed, N. I. (2019). Anesthetics: from modes of action to unconsciousness and neurotoxicity. Journal of Neurophysiology, 122(2), 760–787. https://doi.org/10.1152/jn.00210.2019
Jurič, D. M., Bulc Rozman, K., Lipnik-Štangelj, M., Šuput, D., & Brvar, M. (2022). Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development. Toxins, 14(10). https://doi.org/10.3390/toxins14100720
Kim, J. L., Bulthuis, N. E., & Cameron, H. A. (2020). The Effects of Anesthesia on Adult Hippocampal Neurogenesis. Frontiers in Neuroscience, 14, 588356. https://doi.org/10.3389/fnins.2020.588356
Kraus, C., Wasserman, D., Henter, I. D., Acevedo-Diaz, E., Kadriu, B., & Zarate, C. A., Jr. (2019). The influence of ketamine on drug discovery in depression. Drug Discovery Today, 24(10), 2033–2043. https://doi.org/10.1016/j.drudis.2019.07.007
Kurdi, M. S., Theerth, K. A., & Deva, R. S. (2014). Ketamine: Current applications in anesthesia, pain, and critical care. Anesthesia, Essays and Researches, 8(3), 283–290. https://doi.org/10.4103/0259-1162.143110
Lu, H.-C., & Mackie, K. (2016). An Introduction to the Endogenous Cannabinoid System. Biological Psychiatry, 79(7), 516–525. https://doi.org/10.1016/j.biopsych.2015.07.028
Mlost, J., Bryk, M., & Starowicz, K. (2020). Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. International Journal of Molecular Sciences, 21(22). https://doi.org/10.3390/ijms21228870
Murray, J. B. (1986). Marijuana’s effects on human cognitive functions, psychomotor functions, and personality. The Journal of General Psychology, 113(1), 23–55. https://doi.org/10.1080/00221309.1986.9710540
Rosenbaum, S. B., Gupta, V., Patel, P., & Palacios, J. L. (2023). Ketamine. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK470357/
Silvestro, S., Mammana, S., Cavalli, E., Bramanti, P., & Mazzon, E. (2019). Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials. Molecules , 24(8). https://doi.org/10.3390/molecules24081459
Testai, F. D., Gorelick, P. B., Aparicio, H. J., Filbey, F. M., Gonzalez, R., Gottesman, R. F., Melis, M., Piano, M. R., Rubino, T., Song, S. Y., & American Heart Association Stroke Brain Health Science Subcommittee of the Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; and Council on Peripheral Vascular Disease. (2022). Use of Marijuana: Effect on Brain Health: A Scientific Statement From the American Heart Association. Stroke; a Journal of Cerebral Circulation, 53(4), e176–e187. https://doi.org/10.1161/STR.0000000000000396
The Neuron. (n.d.). Retrieved October 22, 2023, from https://www.brainfacts.org/brain-anatomy-and-function/anatomy/2012/the-neuron
VanDolah, H. J., Bauer, B. A., & Mauck, K. F. (2019). Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clinic Proceedings. Mayo Clinic, 94(9), 1840–1851. https://doi.org/10.1016/j.mayocp.2019.01.003
Wu, L., Zhao, H., Weng, H., & Ma, D. (2019). Lasting effects of general anesthetics on the brain in the young and elderly: “mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. Journal of Anesthesia, 33(2), 321–335. https://doi.org/10.1007/s00540-019-02623-7
Acknowledgement
- University of Calgary, Hotchkiss Brain Institute: Dr. Naweed Syed, Zainab Khan, Fahad Iqbal, and the Syed Laboratory members for their mentorship and facilities
- Dr. Garcia at Webber Academy for her support and guidance