Making Tailing Pond Water Clean Again
Grade 8
Presentation
Hypothesis
I hypothesize that the sand will work the best to filter out the oil from the water. This is because sand is natural and is already known to filter well. Combined with the fact that sand can be found in various places, this would seem to be a very cost-effective solution.
Research
Key terms - Hydrophobic, hydrophilic
Hydrophobic means extremely difficult to get wet.
Hydrophilic is the opposite - it means to be very easily wetted.
Contact angle: The contact angle is defined as the angle between a tangent to the liquid surface and the solid surface at this point [12]. The contact angle of a water droplet on a hydrophilic surface is obtuse, while the contact angle of a water droplet on a hydrophobic surface is acute.
The contact angle of different surfaces [7]:

According to NRDC or Natural Resources Department Council, tailings are poisonous brews of water, sand, silt and petrochemical waste products that are extracted from oil sand mining operations. These ponds have a huge environmental impact. They sicken local communities, poison wildlife, and contaminate Alberta's water resources. [4]
Tailings ponds are problematic due to the hazardous compounds they contain, such as ammonia, mercury, and naphthenic acids. These toxins can harm animals and aquatic life, and the ponds often leak, contaminating the surrounding land and groundwater. Additionally, the ponds require long-term containment, with over 830 million cubic meters needing management. This makes it hard for wildlife to survive, especially birds who may be exposed to danger when coming into contact with the ponds. [5] (Energy Education)
There are 3 oil sands deposits in Alberta, each containing a number of tailing ponds. They are the Athabasca Oil Sands, Peace River Oil Sands, and Cold Lake Oil Sands. [13]
During bitumen extraction, an enormous volume of tailings waste is generated. This waste requires a vast storage area, which can negatively impact wildlife habitat. To mitigate the environmental impact, bird deterrence measures have been developed to keep birds away from the ponds. These measures involve the use of air cannons to scare birds away. Companies have been given targets to reduce tailings and set dates for pond closure and reclamation. Timelines are being developed to handle fluid tailings so that they can be processed at the same speed as they are being created. All of these measures are to prevent tailings growth. [5] (Energy Education)
In April 2008, 1,600 ducks were found dead after landing on a tailing pond north of Fort McMurray, Alberta. In the days following the animal disaster, both federal and provincial officials promised to take action against Syncrude, with fines of up to $1 million. [6] (Ecojustice)
[10]
[11]
We have 3 different filtering prototypes. Filter paper, filter paper + sand, and lastly sand.
Molecular Structure of Canola Oil: [8]
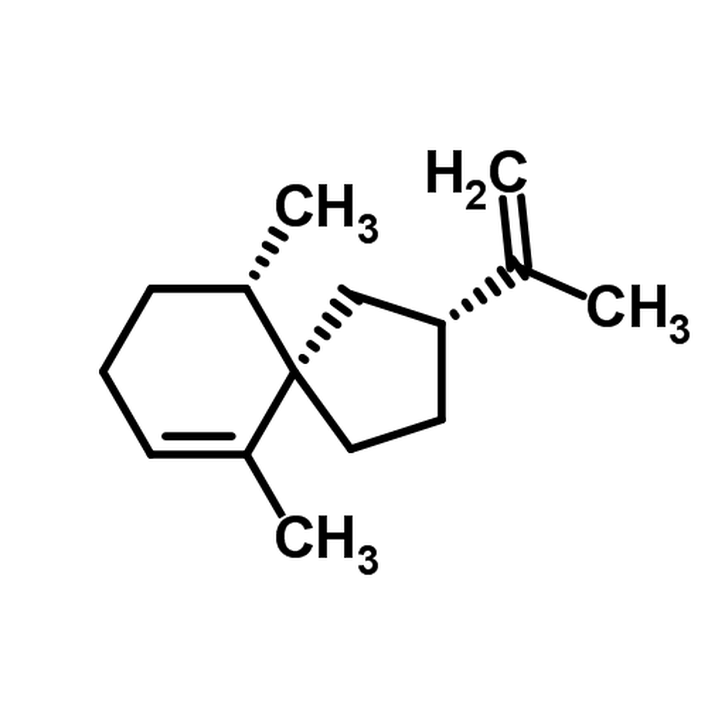
Molecular Structure of Water: [9]
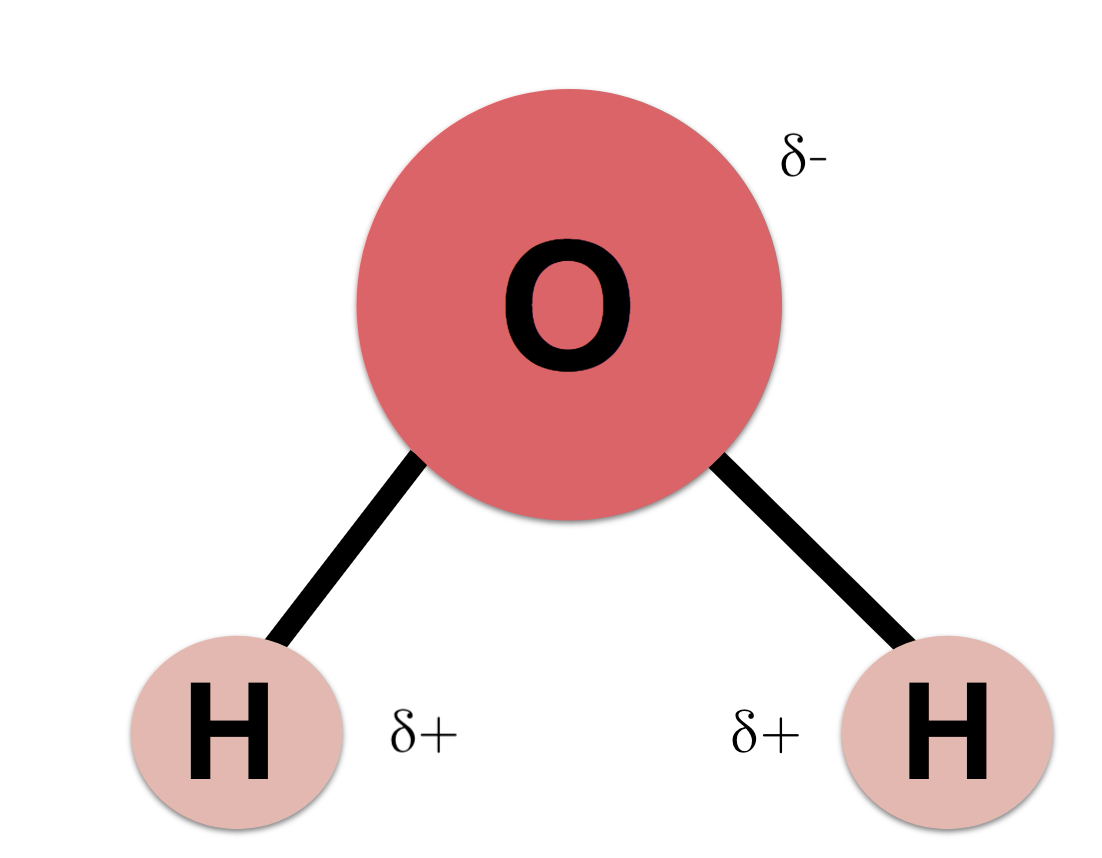
Variables
Manipulated Variables - The filter material (filter paper and sand)
Controlled variables - Water, Oil, Beaker, Metal Mesh, Metal Stand
Responding Variables - Efficiency of filtration
Procedure
WATER/OIL FILTRATION WITH FILTER PAPER PROCEDURE:
1. Drill holes of 1-2mm into a bottle cap (I used a Gatorade bottle)
2. Cut the bottle 3/4 of the way to create a funnel-like object
3. Cut out a piece of metal mesh of appropriate size (size of the inner circle of the cap), this is to ensure the cap can still be put back on the bottle without damaging the mesh
4. Put the metal mesh on the cap's inner circle and ensure it's at a suitable size
- The best size is when it can fit in the inner circle, but it still won't fall out when shaken
5. Cut the filter paper to around the same size as the metal mesh
6. Put the filter paper right above the metal mesh - it should also fit in the inner circle of the cap
7. Screw the cap back onto the bottle
8. Pre-wet the filter paper, whether by taking the paper out and wetting it or pouring water into the bottle to test out the cap and wet the filter paper at the same time
9. Assemble a metal stand to hold the bottle in place
10. Put a beaker underneath the bottle to catch any falling liquid
11. Pour in a 1:1 ratio of oil and water into the bottle (make sure that the beaker doesn't contain any excess liquid before this step)
12. Note down any observations
WATER/OIL FILTRATION WITH SAND PROCEDURE:
1. Repeat steps 1-4
2. Fill the bottle cap with a layer of sand up to a point where you can still close the bottle
3. Screw the cap back onto the bottle
4. Put the bottle onto the metal stand (optional)
5. Put a beaker/container underneath the bottle to catch any falling liquid
6. Pre-wet the sand (optional)
7. Fill a beaker with a 1:1 ratio of oil and water
8. Pour the mixture into the bottle
WATER/OIL FILTRATION WITH FILTER PAPER AND SAND PROCEDURE:
1. Repeat step 1-6 from the first experimental procedure
2. Add sand on top of the filter paper in the bottle cap up to a point where you can still close the bottle
3. Screw the cap back onto the bottle
4. Put the bottle onto the metal stand (optional)
5. Put a beaker/container underneath the bottle to catch any falling liquid
6. Pre-wet the sand + filter paper
7. Fill a beaker with a 1:1 ratio of oil and water
8. Pour the mixture into the bottle
Observations
Filter Paper Attempt #1:
- All of the water rushed out very quickly
- Towards the middle, all that was left was oil
- It poured out slowly but in big droplets
- I didn't manage to filter it, however
- I will attempt to add more layers of filter paper, and perhaps that will solve the problem
- After ~4 minutes, the oil reached the 5mL mark
- The oil was much slower than the water due to its higher viscosity
Filter Paper Attempt #2:
- Very similar to attempt 1
- I wanted to try one layer of filter paper again to see what else I could change
- I realized that when the liquid from the bottle slowed down, the oil would soon be coming out.
- A solution to the problem of all liquid falling out would be to quickly switch the containers when the water turned to oil, this way the water would be in one container, and the oil In another
- This time, I ended up with almost exactly 20mL of oil and 20mL of water
Filter Paper Attempt #3:
- I realized that the problem was that the filter paper was cut too small, so the oil could escape from the edges and fall out
- To fix this, I decided to leave my filter paper uncut and used 2 layers instead of one
- This fixed the problem and the water escaped through easily, leaving the oil stuck behind in the bottle
Filter Paper Attempt #4
- Water and oil were filtered with water rushing out and oil remaining in the bottle from being blocked by the filter paper
- This was extremely similar to attempt #3, and the only reason I did this was to check if attempt #3 was able to be repeated
Filter Paper Attempt #5
- Same as attempt #3 and #4, water and oil were filtered with water in the beaker and oil remaining in the bottle
- This was also to see if attempt #3 was a fluke or if it was a consistent result
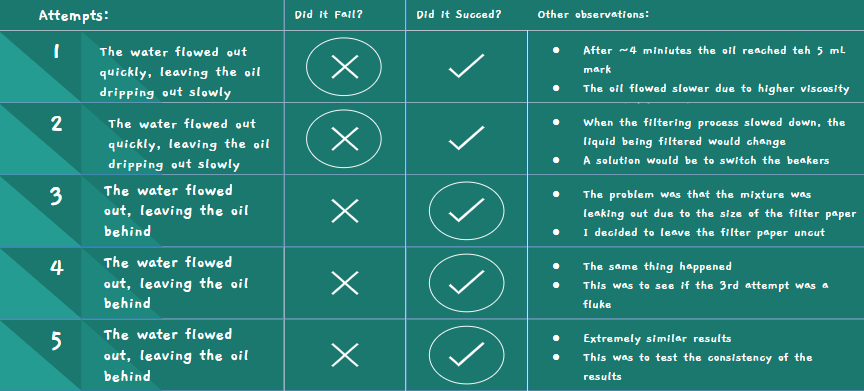
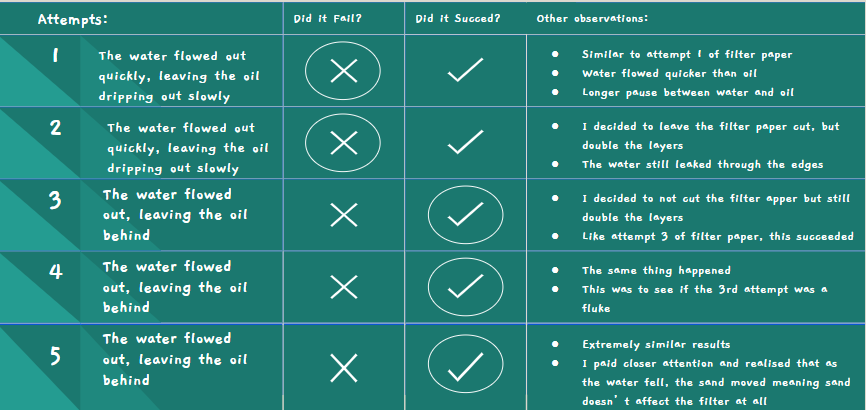
Sand Attempt #1
- Once again, water rushed out quicker than oil
- I had to move the beakers to filter the oil and water separately
- It filtered better than the paper since there was a longer time between the water and oil dripping out
Sand Attempt #2
- This was to see if I would get the same result
- Once again, the water rushed out quickly and the oil followed behind, still dripping slowly
Sand Attempt #3
- This time, I watched closely to see what the problem was
- I realized that as the water fell on the layer of sand, it would move the sand away so that there would be space for the oil to fall through the sand unfiltered
Sand Attempt #4
- This time, I tried to put two layers of metal mesh
- One below the sand, and one above
- This still didn't work, as the bottle cap was unable to be put on tightly due to the amount of sand in the bottle cap
- The water and oil just escaped through the sides of the bottle
Sand Attempt #5
- This time, I wrapped raw materials around the sand to try to prevent the water and oil from leaking through the sides
- This still didn't work, as the water and oil just leaked through from between the raw materials and the bottle
- To filter this, I still had to switch out the beakers
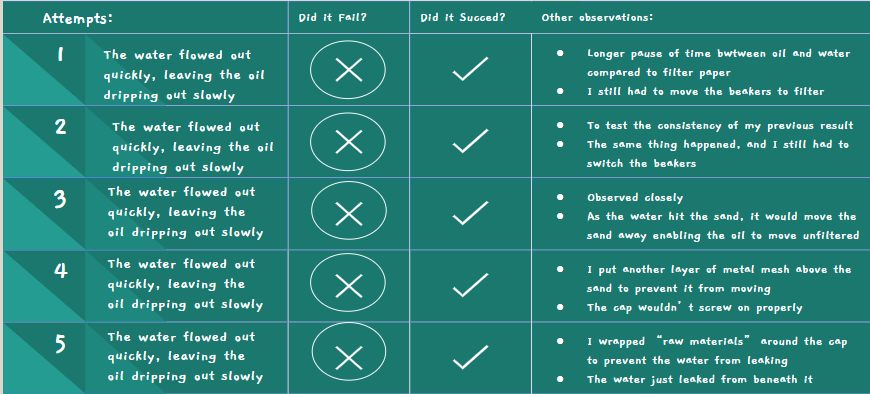
Filter Paper and Sand Attempt #1
- Similar to attempt #1 of the filter paper experiment
- The water flowed out quickly, and in the end, the oil was just coming out slowly in big droplets
- A little bit easier to do than filter paper attempt #1, since there was a longer time in between the water and oil used to switch the beakers
- Also a bit easier due to previous experience with switching the beakers
Filter Paper and Sand Attempt #2
- After the last attempt failed, I decided to double the filter paper but still cut the paper to see if it was due to the doubling that filter paper #3 was able to succeed
- Same results as #1, the oil and water stilled both leaked out in a similar fashion to before
Filter Paper and Sand Attempt #3
- This time, I decided to do the same thing as before, but leave the filter paper uncut
- I still doubled it just to make sure
- This time, it was a success
- The water quickly rushed out, leaving the oil trapped in the bottle
- Success!
Filter Paper and Sand Attempts #4 & #5
- I put them together because they were so similar
- Very similar to attempt #3
- I only did these to ensure that attempt #3 wasn't a fluke
- I paid closer attention to the sand this time
- As the water hit the sand, it got pushed around a lot, meaning if it was just sand it wouldn't have worked
- The filter paper did most of the work since both the water and the oil couldn't move it at all
I also tested out the contact angles of water and oil on filter paper, and the images are attached below:


Analysis
Efficiency:
From the many attempts I made in my experiment, I ultimately deduced that sand does not affect the filtration of the oil-water mixture at all. The filter paper was ultimately a huge success and was able to filter the mixture, letting the water rush out to the beaker and leaving the oil in the bottle. This means that filter paper is more effective in filtering than sand.
Cost:
The cost of 100 pieces of filter paper is $15.99 (without tax) or around 16 cents per piece.
The cost of 5 lbs of sand is $7.97 (without tax) and the cost of the amount of sand used in each of my experiments (2g) is 0.7 cents (453.592 g in a lb, 453.592 x 5 = 2267.96 g in 5 lbs, 7.97/2267.96x2 ≈ 0.007, so the cost of 2g of sand is ≈ 0.7 cents).
This means that the sand is a lot cheaper than the filter paper, filter paper is almost 23 times the cost of sand for my experiments!
Overall:
Filter paper wins in the efficiency category, with filter paper + sand coming in second. Sand wins in the cost category, with filter paper coming in second. Although they both have their benefits, I think that actually being able to filter is the most important, so filter paper is the best filter for oil-water filtering.
Why it works:
The filter paper is able to filter the oil-water mixture because of its hydrophilic nature. The filter paper is hydrophilic, while the oil is hydrophobic, so the filter paper will not let the oil go through. Due to its hydrophilic nature, the filter paper will naturally let the water through, thus leaving the oil behind.
Conclusion
Filter paper is the best filtration material out of the 3 that I tested. Albeit much more expensive than sand, it is so much more efficient in filtering. My hypothesis was proven wrong through this experiment since even though sand can be found everywhere and is extremely cheap, its filtration quality in my experiment isn’t the best. However, my small-scale experimental setup may limit the sand filtration potential, but given the observations and analysis of this project, filter paper is the best filtration material in my experiment.
Application
This information can be applied to oil spills and tailing ponds and in the future, tailings. Both oil spills and tailing ponds are incredibly dangerous to humans and animals alike. By filtering the oil from the water, we’ll be able to lessen the danger of them. Although my experiment was done with light oil (found in some oil spills and tailing ponds) it can be done with heavy oil (found in other oil spills and tailings). This is due to the hydrophilic nature of filter paper and the hydrophobicity of both heavy oil and light oil.
Sources Of Error
- The full potential of filtering with sand hasn’t been covered by my experiment, as I couldn’t get the sand to fit into the bottle with enough space to screw on the cap.
- The sand weight was just an estimate, since I wasn’t putting exactly 2 g every single time
- The holes on my bottle weren’t the most even, and that could have affected how the water came out
- My pours could’ve been better paced to maintain a steady flow. This could’ve affected how fast the water went out or could’ve added extra pressure for the oil to go out
Citations
[2] https://www.frontiersin.org/articles/10.3389/fchem.2020.00507/full
[3] https://energyeducation.ca/encyclopedia/Oil_sands_tailings_ponds
[5] https://ecojustice.ca/file/polluters-pay-after-duck-deaths-on-syncrude-tailings-pond/
[6] https://www.capp.ca/explore/tailings-ponds/
[8] http://chembathbomb.weebly.com/canola-oil.html
[10] https://www.nationalobserver.com/2017/11/03/analysis/how-syncrude-and-friends-benefitted-creative-sentence-2010-oilsan ds-duck-deaths
[11] https://kids.nationalgeographic.com/animals/birds/facts/mallard-duck
[12] https://www.brighton-science.com/what-is-contact-angle#:~:text=The%20contact%20angle%20is%20defined,the%20contact%20angle%20becomes%20smaller.
[13] https://www.aer.ca/providing-information/by-topic/tailings
Acknowledgement
- I acknowledge that my mom helped me with assembling the metal stand and helped me order the materials for this experiment.
- I acknowledge that my teacher, Ms. Lai, helped me sign up for CYSF and provided unlimited support for us students.
- Finally, I acknowledge CYSF, sponsors for CYSF, and all of the judges for taking the time to host, donate, and judge for CYSF. Thank you all because without you, this experiment wouldn't be possible.

